TBC1D32 variants disrupt retinal ciliogenesis and cause retinitis pigmentosa
JCI Insight 2023 Nov 08;821:. doi: 10.1172/jci.insight.169426.
Bocquet B, Borday C, Erkilic N, Mamaeva D, Donval A, Masson C, Parain K, Kaminska K, Quinodoz M, Perea-Romero I, Garcia-Garcia G, Jimenez-Medina C, Boukhaddaoui H, Coget A, Leboucq N, Calzetti G, Gandolfi S, Percesepe A, Barili V, Uliana V, Delsante M, Bozzetti F, Scholl HP, Corton M, Ayuso C, Millan JM, Rivolta C, Meunier I, Perron M, Kalatzis V.
Click here to view article at JCI Insight.
Click here to view article on PubMed.
Click here to view article on Xenbase.
Abstract
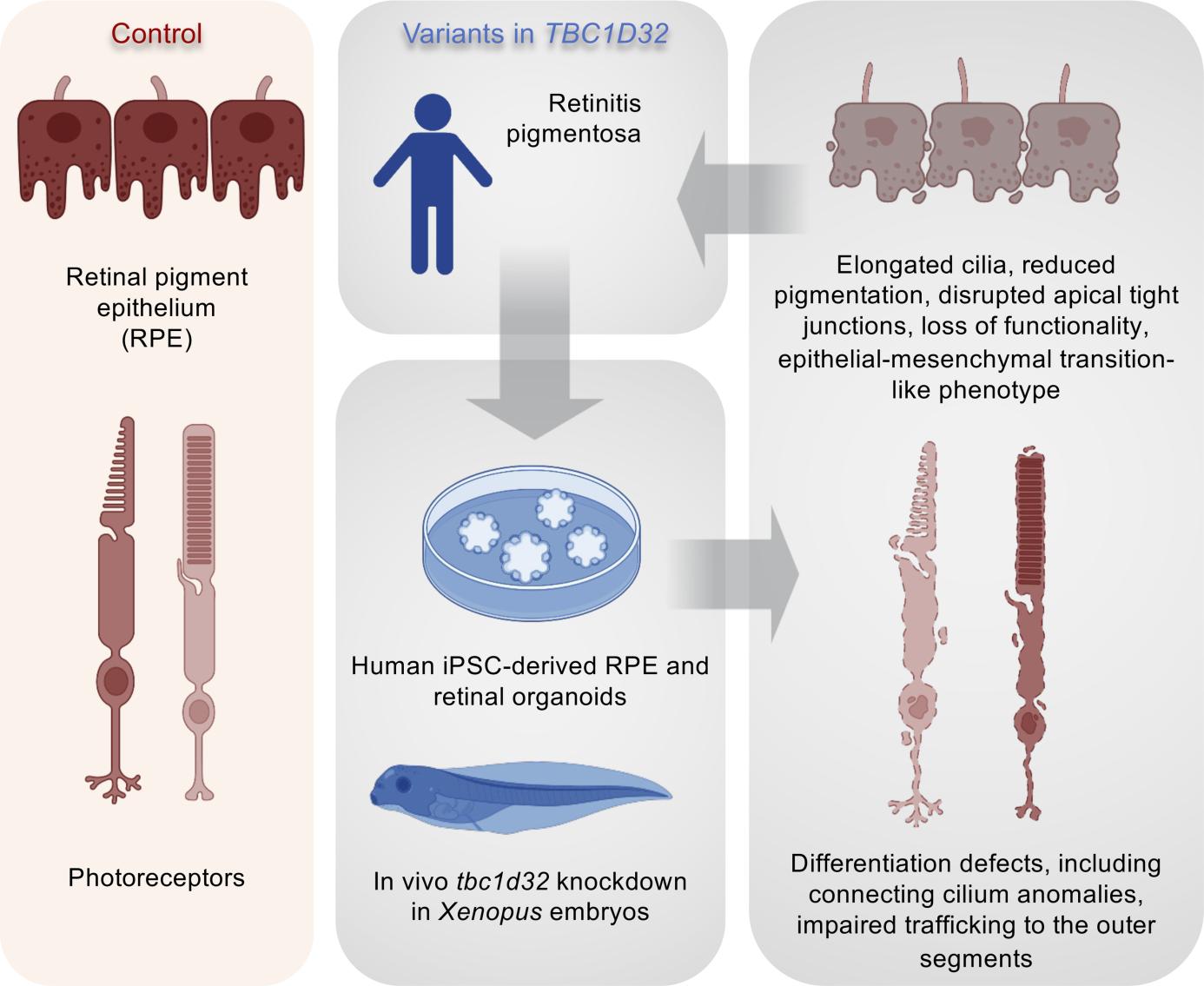
Retinitis pigmentosa (RP) is the most common inherited retinal disease (IRD) and is characterized by photoreceptor degeneration and progressive vision loss. We report 4 patients presenting with RP from 3 unrelated families with variants in TBC1D32, which to date has never been associated with an IRD. To validate TBC1D32 as a putative RP causative gene, we combined Xenopus in vivo approaches and human induced pluripotent stem cell-derived (iPSC-derived) retinal models. Our data showed that TBC1D32 was expressed during retinal development and that it played an important role in retinal pigment epithelium (RPE) differentiation. Furthermore, we identified a role for TBC1D32 in ciliogenesis of the RPE. We demonstrated elongated ciliary defects that resulted in disrupted apical tight junctions, loss of functionality (delayed retinoid cycling and altered secretion balance), and the onset of an epithelial-mesenchymal transition-like phenotype. Last, our results suggested photoreceptor differentiation defects, including connecting cilium anomalies, that resulted in impaired trafficking to the outer segment in cones and rods in TBC1D32 iPSC-derived retinal organoids. Overall, our data highlight a critical role for TBC1D32 in the retina and demonstrate that TBC1D32 mutations lead to RP. We thus identify TBC1D32 as an IRD-causative gene.
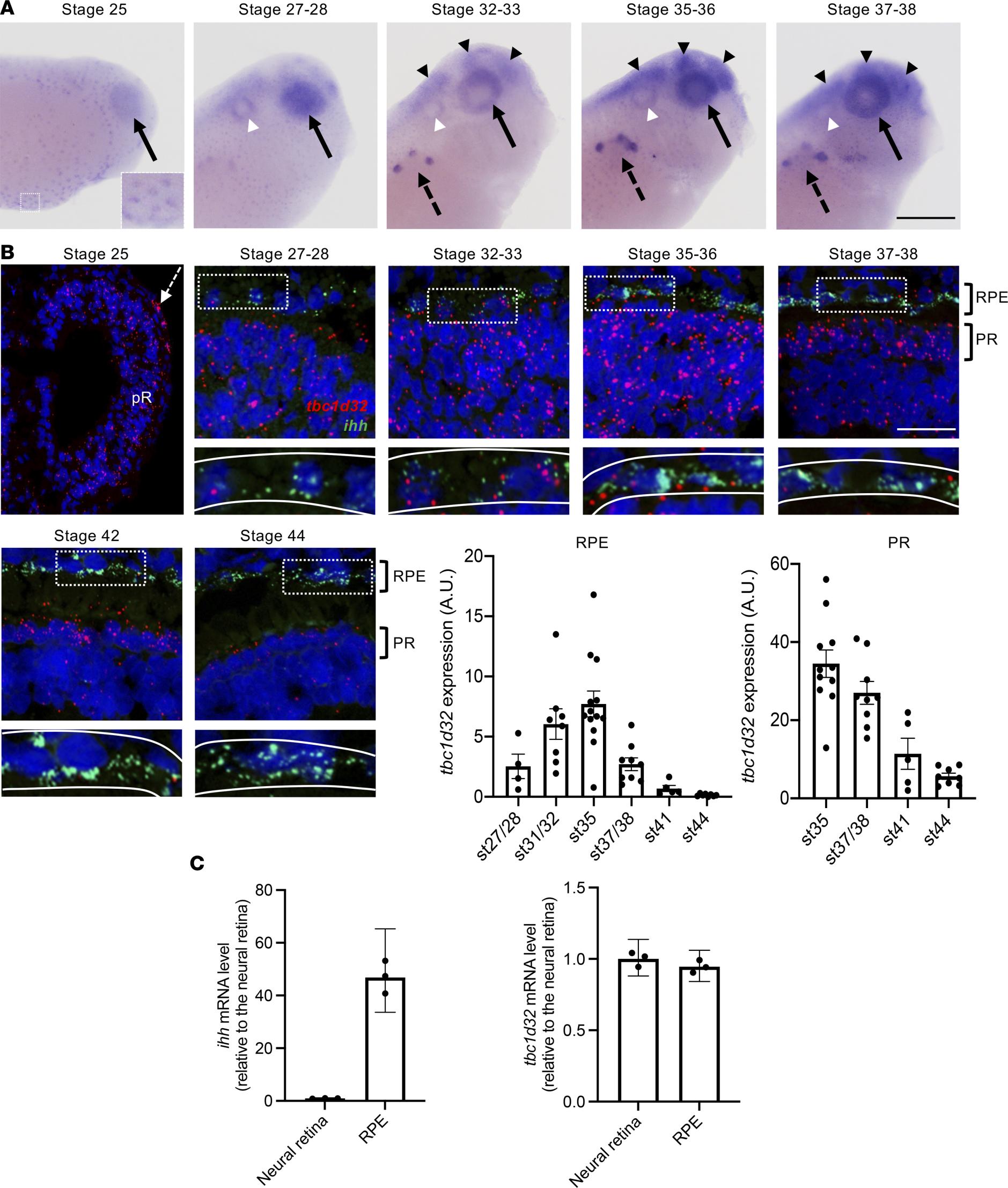
Figure 2. tbc1d32 expression during Xenopus development and retinogenesis. (A) Lateral views of Xenopus embryo heads (anterior to the right) following tbc1d32 whole-mount in situ hybridization. Enlargement: at stage 25, epidermal cells are stained. tbc1d32 expression is also detected in the eye (black arrows) and later in the pronephric nephrostomes (black dotted arrows), brain (black arrowheads), and otic vesicle (white arrowheads). (B) RNAscope in situ hybridizations for tbc1d32 and ihh on cryostat sections. A region of RPE cells, delineated by white dotted boxes in the upper panels, is enlarged in the lower panels, where the RPE layer is outlined (white lines). Scatterplots with bars represent the quantification of tbc1d32 expression in the RPE or photoreceptor layer at each stage (st). Data are represented as mean SEM. Each dot represents 1 retina. Scale bars = 400 m in A, 50 m in B. AU, arbitrary units; pR, presumptive retina; RPE, retinal pigment epithelium; PR, photoreceptors. (C) qPCR analysis of ihh and tbc1d32 expression in neural retina and RPE tissues, dissected at stage 3536. ihh serves as a specific RPE marker. Data are represented as geometric mean with 95% CI; n = 3 technical replicates.
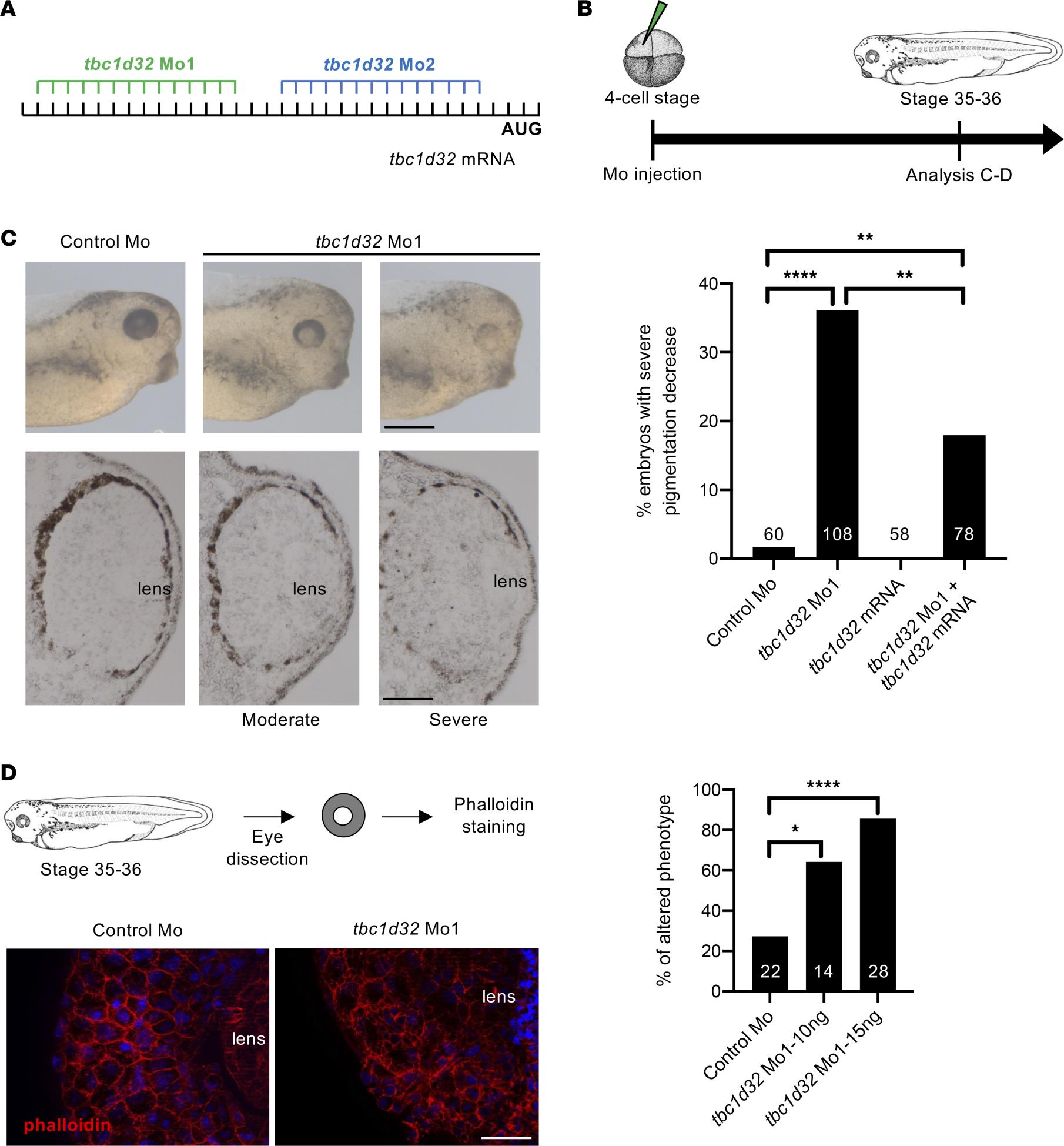
Figure 3. Xenopus RPE phenotype following tbc1d32 knockdown. (A) Mo1 and Mo2 target sequences located in the 5 untranslated region of tbc1d32 mRNA. (B) Diagram of the experimental design. (C) Upper panels, lateral views of 1 control and 2 morphant embryo heads with moderate and severe phenotypes (anterior to the right). Lower panels, transverse retinal sections of control and morphant embryos (dorsal side up). The bar plot represents the percentage of embryos with a severe decrease in pigmentation among control (control Mo), morphant (tbc1d32 Mo1), tbc1d32 mRNA-injected, and tbc1d32 Mo1/tbc1d32 mRNA coinjected groups. The total number of embryos analyzed per condition is indicated in each bar. **P < 0.01; ****P < 0.0001; Fishers exact test. (D) Phalloidin staining of filamentous actin on dissected eyes of control or morphant Xenopus embryos. The bar plot represents the proportion of eyes with an altered distribution of F-actin for each condition. The total number of eyes analyzed per condition is indicated in each bar. *P < 0.05; ****P < 0.0001; Fishers exact test. Scale bars = 400 m for whole mounts and 60 m for sections in C, 20 m in D.
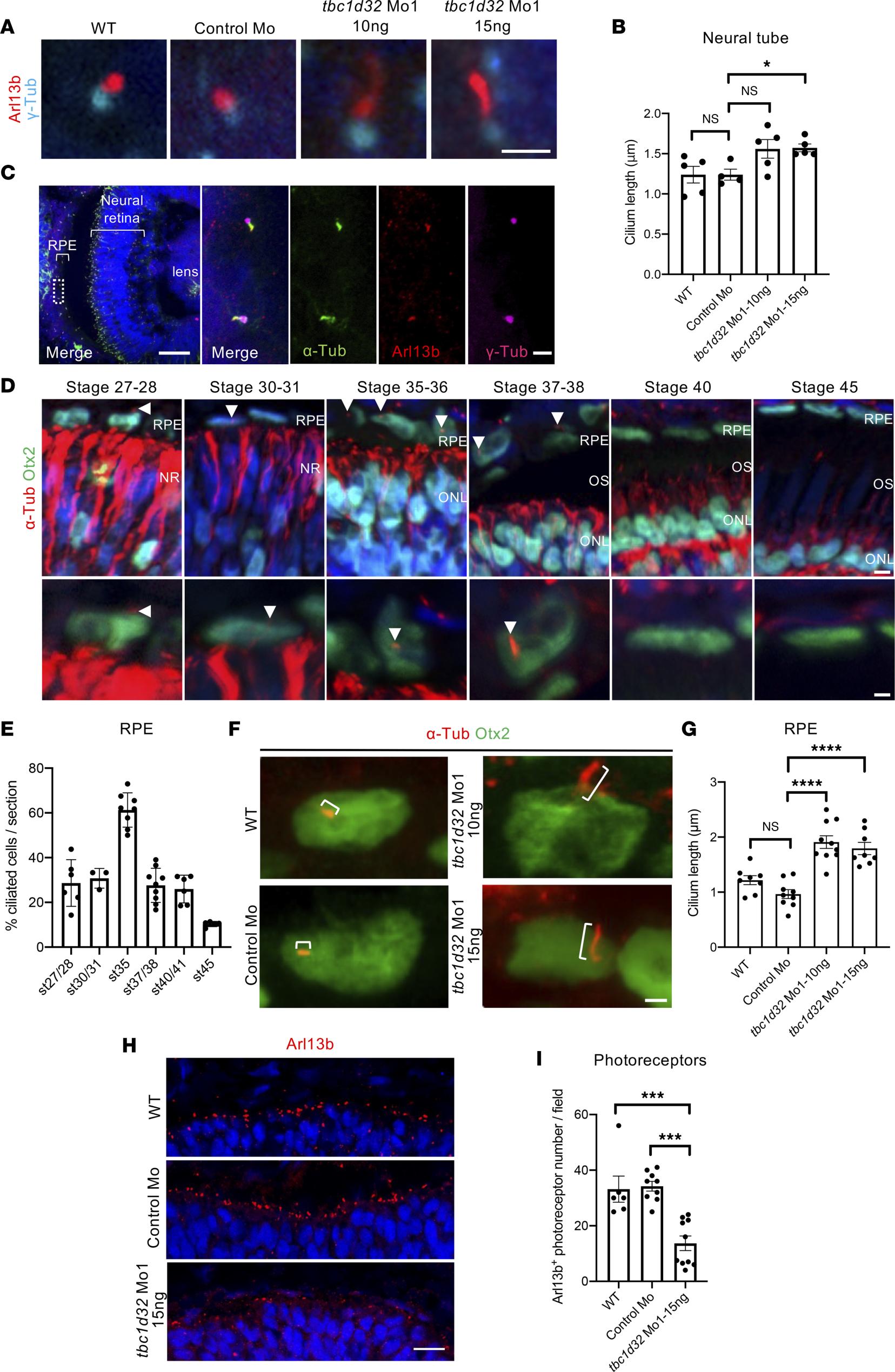
Figure 5. Xenopus ciliogenesis following tbc1d32 knockdown. (A) Arl13b and -Tubulin (-Tub) immunolabeling on neural tube sections of stage 3536 wild-type (WT), control Mo, or tbc1d32 Mo1 embryos. (B) Scatterplot shows the mean length of neural tube cilia. Each dot corresponds to the mean length for 1 embryo. (C) Immunolabeling of cilia markers, acetylated -Tubulin (-Tub), Arl13b, and -Tub, on retinal sections of stage 3536 WT embryo. The right panels show an enlargement of the area delineated by a white dotted box in the left panel. (D) -Tub immunolabeling of RPE cilia (arrowheads) at different stages. Sections are costained with Otx2 to identify RPE cells, which are enlarged in the lower panels. NR, neural retina; ONL, outer nuclear layer; OS, outer segment. (E) Proportion of ciliated cells among RPE cells at different stages (st). (F) -Tub and Otx2 immunolabeling showing primary RPE cilia (brackets) on retinal sections of stage 35 WT, control Mo, or tbc1d32 Mo1 embryos. (G) Scatterplot with bars showing the mean cilia length in RPE cells. Each dot corresponds to the mean length for 1 embryo. (H) Immunolabeling of Arl13b showing the photoreceptor connective cilium on retinal sections of stage 35 WT, control Mo, or tbc1d32 Mo1 embryos. (I) The scatterplot shows the mean number of Arl13+ ciliated photoreceptors in 1 field of the central retina. Each dot corresponds to 1 embryo. All data are represented as mean SEM. *P < 0.05, ***P < 0.001, ****P < 0.0001; 2-tailed Mann-Whitney test. Scale bars = 2 m in A and F, 50 m and 2 m for enlargements in C, 5 m and 2 m for enlargements in D, 25 m in H.
Adapted with permission from the American Society for Clinical Investication on behalf of the JCI Insight: Bocquet et al. (2023). TBC1D32 variants disrupt retinal ciliogenesis and cause retinitis pigmentosa. JCI Insight 2023 Nov 08;821:. doi: 10.1172/jci.insight.169426.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2024-02-13