Production and characterization of monoclonal antibodies to Xenopus proteins
Horr B , Kurtz R , Pandey A , Hoffstrom BG , Schock E , LaBonne C , Alfandari D .
Development 2023 Feb 14; doi: 10.1242/dev.201309.
Click here to view article at Development.
Click here to view article on PubMed.
Click here to view article on Xenbase.
Abstract
Monoclonal antibodies are powerful and versatile tools that enable the study of proteins in diverse contexts. They are often utilized to assist with identifying subcellular localization and characterizing the function of target proteins of interest. However, because there can be considerable sequence diversity between orthologous proteins in Xenopus and mammals, antibodies produced against mouse or human proteins often do not recognize Xenopus counterparts. To address this issue, we refined existing protocols to produce mouse monoclonal antibodies directed against Xenopus proteins of interest. Here we describe several approaches for the generation of useful mouse anti-Xenopus antibodies to multiple Xenopus proteins and their validation in various experimental approaches. These novel antibodies are now available to the research community through the Developmental Study Hybridoma Bank (DSHB).
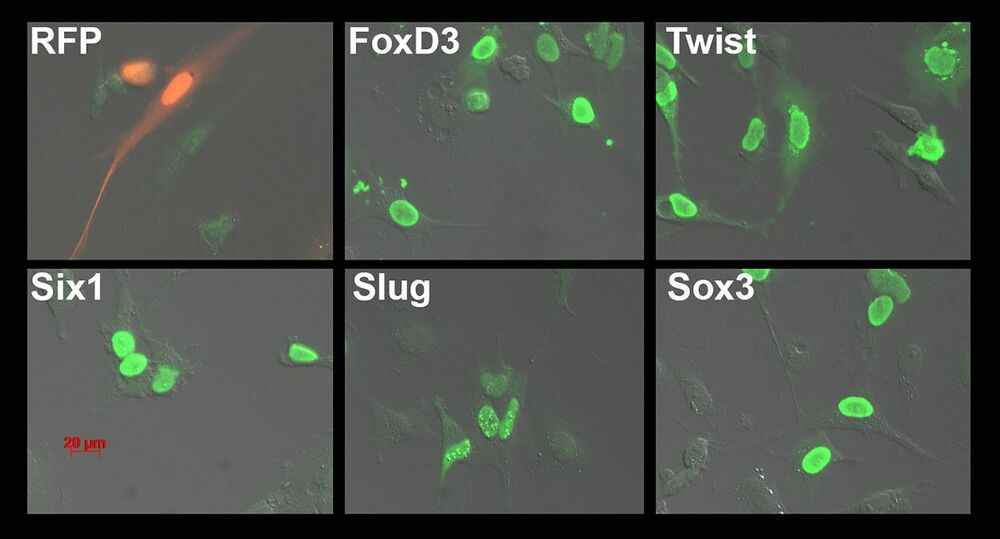
Fig. 1. Immunofluorescence testing of serum. The serum from mouse M3 immunized with FoxD3-Flag, Twist-Flag, Six1-Flag, Slug-Flag and Sox3-Flag produced in Hek293T cells was tested by immunofluorescence on XTC cells transfected with each of the target or RFP-Flag as a negative control. A secondary Alexa 488-conjugated anti-mouse antibody was used to visualize the mouse primary antibodies (green). Notice the green nuclei in each of the transfections, with some non-transfected cells lacking signal.
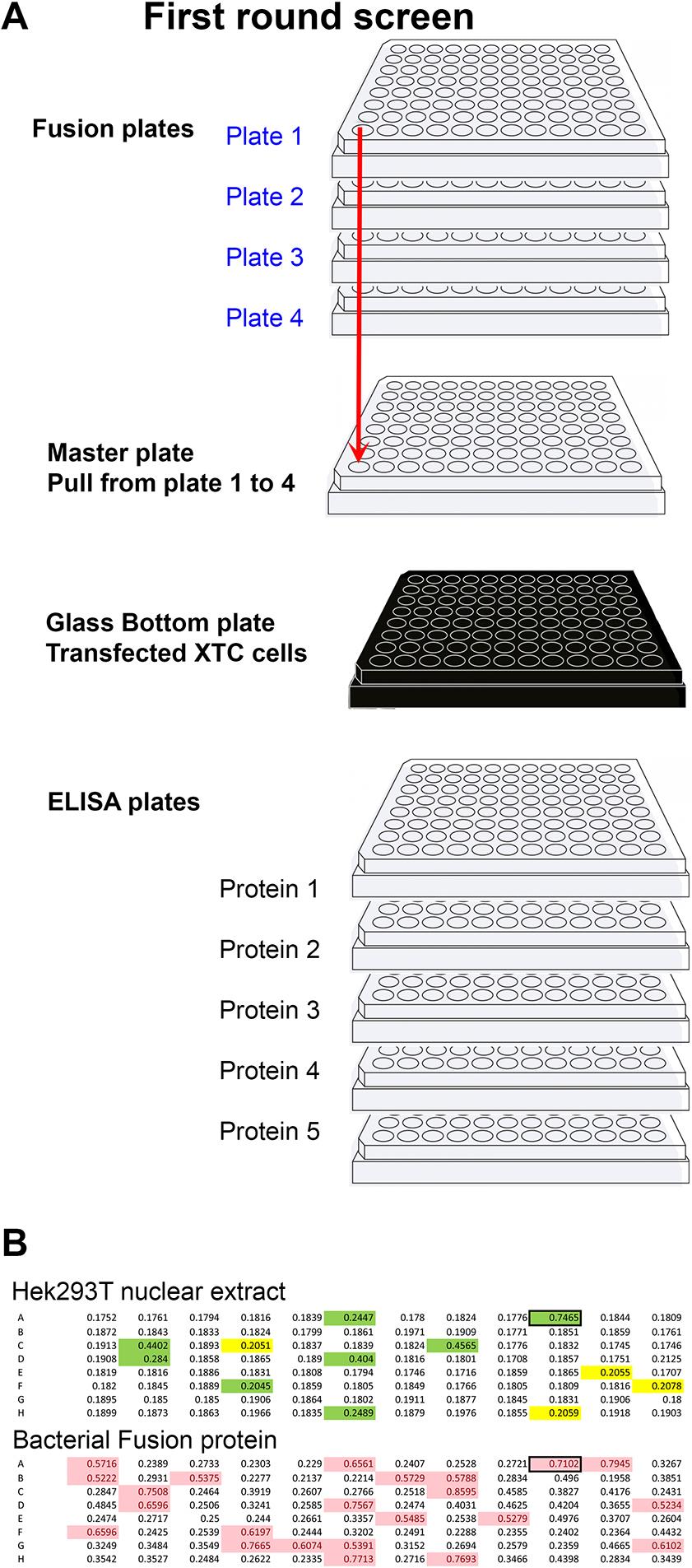
Fig. 2. Primary screening method. (A) Diagram representing the strategy used to rapidly identify wells producing antibodies to multiple targets at once. Four plates per fusion were used. Thirty microlitres were taken from each well and placed in the master plate (total volume 120 µl). The master plate was diluted with 200 µl of TBST (TBS with 0.1% Tween 20). From the 320 µl of the master plate, 50 µl was added to each of the five ELISA plates plus the glass-bottom plate containing XTC cells transfected with each of the five targets. Simultaneously, 50 µl of supernatant was added to each well of five ELISA plates, each coated with individual proteins. Wells positive in either or both assays (A1 to H12) were then tested on each of the original plates (blue) to identify the plate number (deconvolution, 1A1 to 4H12). (B) Primary screen by ELISA using the cytoplasmic domain of Adam13/33 expressed in Hek293T cells (top) and his-tag bacterial fusion protein (bottom). The mice were immunized using the bacterial fusion protein. Highlighted wells are above the chosen background (0.2 for Hek293T and 0.5 for bacterial fusion protein). Wells highlighted in green are common to both ELISA and were selected for further screening.
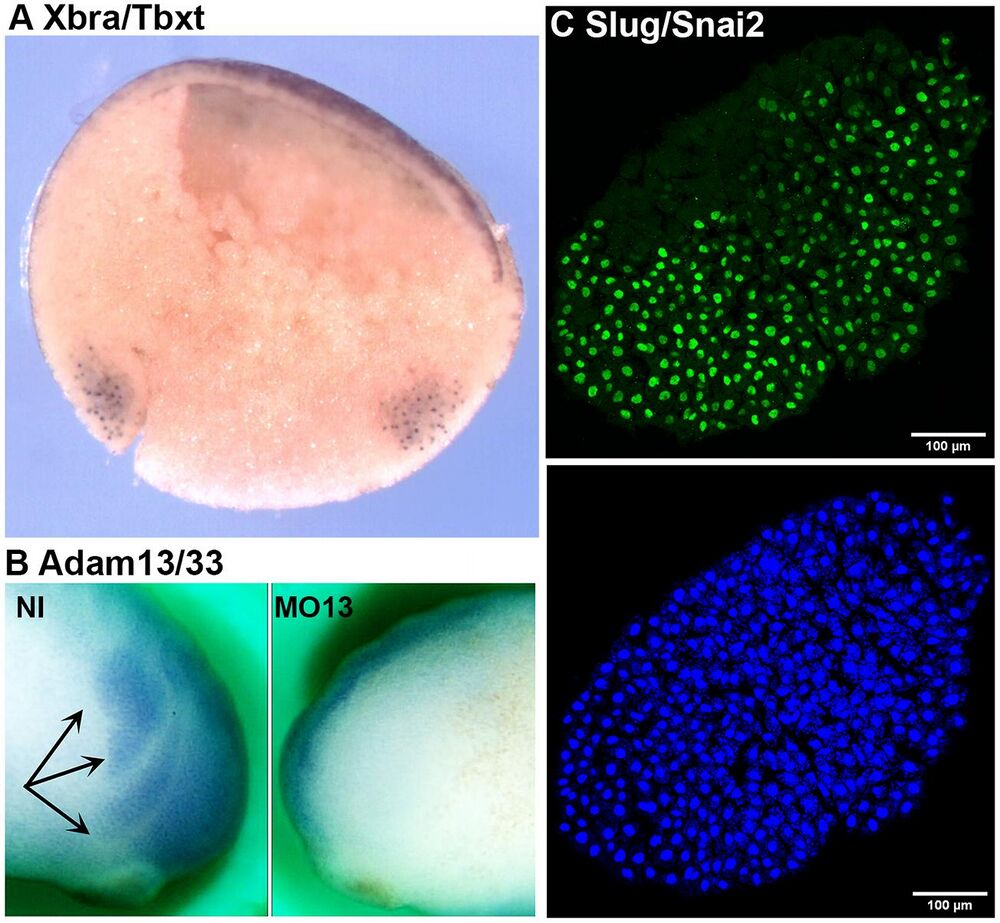
Fig. 5. Immunostaining. (A) Whole-mount immunostaining using DA12H2xbra on bisected gastrula (Xbra/Tbxt). The dorsal side is to the left, and the animal pole is up. The antibody stains the nuclei in both the dorsal and ventral marginal zones (mesoderm). (B) Whole-mount immunostaining using a monoclonal antibody to Adam13/33 (DA8E6adam13) on embryos injected at the two-cell stage with a morpholino that blocks translation of Adam13. The cranial neural crest cells (black arrows) are visible on the non-injected (NI) side, but are absent on the injected side (MO13). (C) Immunofluorescence of a cranial neural crest explant using mAb DA1A8slug (green) and counterstained with DAPI (blue). Note that most of the nuclei are stained for Slug/Snai2 except for a small section on the left of the explant (dorsal) that is likely to be composed of neural plate cells.
Reproduced/adapted with permission from Springer Nature on behalf of Nature Communications: Horr et al. (2023). Production and characterization of monoclonal antibodies to xenopus proteins. Development 2023 Feb 14; doi: 10.1242/dev.201309.
Last Updated: 2023-04-05