Spinal cord regeneration in Xenopus laevis
Edwards-Faret G , Muñoz R , Méndez-Olivos EE , Lee-Liu D , Tapia VS , Larraín J
Nat Protoc. February 1, 2017; 12 (2): 372-389.
View article at Nature Protocols
View article on Pubmed
View article on Xenbase
Abstract
Edwards-Faret et al. present a protocol for the husbandry of Xenopus laevis tadpoles and froglets, and procedures to study spinal cord regeneration. This includes methods to induce spinal cord injury (SCI); DNA and morpholino electroporation for genetic studies; in vivo imaging for cell analysis; a swimming test to measure functional recovery; and a convenient model for screening for new compounds that promote neural regeneration. These protocols establish X. laevis as a unique model organism for understanding spinal cord regeneration by comparing regenerative and nonregenerative stages. This protocol can be used to understand the molecular and cellular mechanisms involved in nervous system regeneration, including neural stem and progenitor cell (NSPC) proliferation and neurogenesis, extrinsic and intrinsic mechanisms involved in axon regeneration, glial response and scar formation, and trophic factors. For experienced personnel, husbandry takes 1-2 months; SCI can be achieved in 5-15 min; and swimming recovery takes 20-30 d.
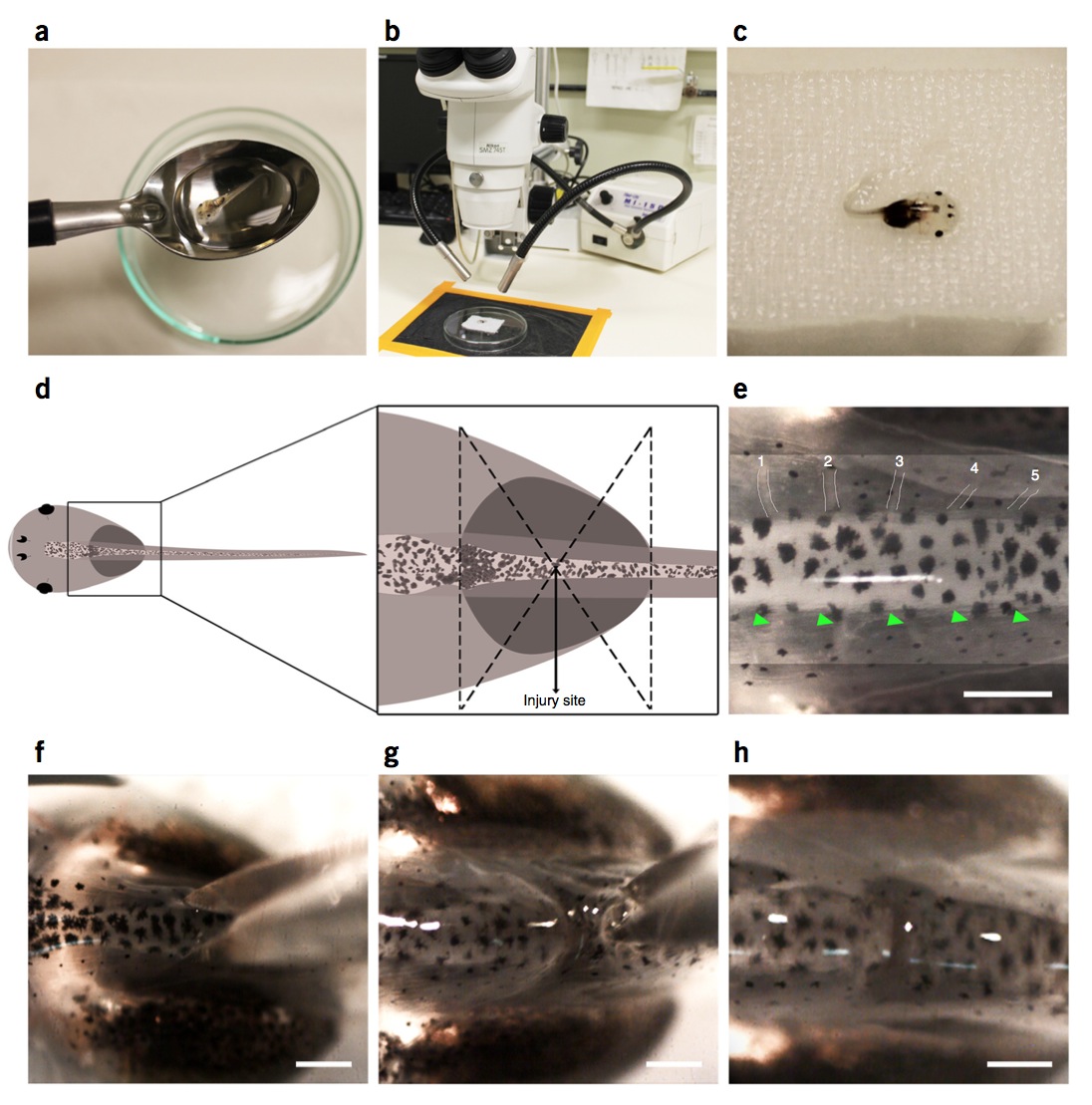
Figure 1 | Spinal cord transection surgery in stage 50 larvae. (a) Stage 50 larvae are transferred from an anesthetic bath to a gauze using a spoon to avoid any damage. (b,c) Larvae are placed dorsal side up on the gauze under the dissecting microscope. (d) Schematic diagram showing location of the injury site at the thoracic level of the spinal cord. (e) Identification of the third and fourth peripheral nerves by white lines or green arrowheads. (f) Scissors position right before transection. (g) Straight cut through skin, muscle and spinal cord. (h) Larva after spinal cord transection. Scale bars, 200 μm. Spinal cord injury in larvae was performed in compliance with the institutional bioethics and biosafety committee at the Pontificia Universidad Catolica de Chile.
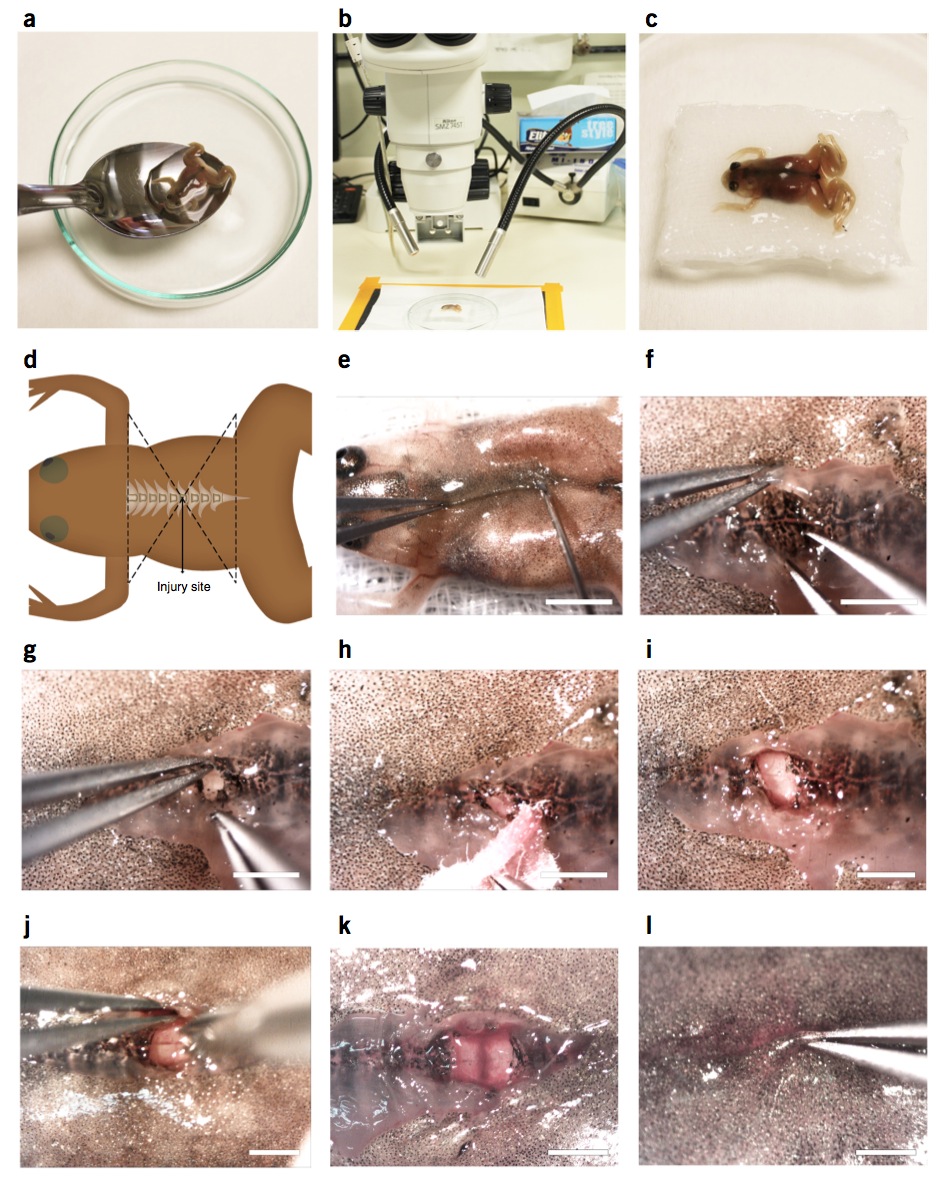
Figure 2 | Spinal cord transection surgery in stage 66 froglets. (a) Stage 66 froglets are transferred from anesthetic to a gauze using a spoon to avoid any damage. (b,c) Froglets are placed dorsal side up on the gauze under the dissecting microscope. (d) Schematic diagram showing the location of the injury site at the thoracic level of the spinal cord. (e) First incision in the skin and muscle with the tip of a 23-gauge needle. (f) Skin and muscle separation to access the dorsal region of the vertebrae. (g) Laminectomy of one vertebra using forceps. (h) Blood should be cleaned with tissue paper. (i) Spinal cord is exposed to proceed with spinal cord transection. (j) Incision across the spinal cord using microdissection scissors. (k) Spinal cord incision. (l) Closure of the injury extending the muscle and then the skin from one side over the other. Scale bars, 5 mm (e); 1.5 mm (f–l). Spinal cord injury in froglets was performed in compliance with the institutional bioethics and biosafety committee at the Pontificia Universidad Catolica de Chile.
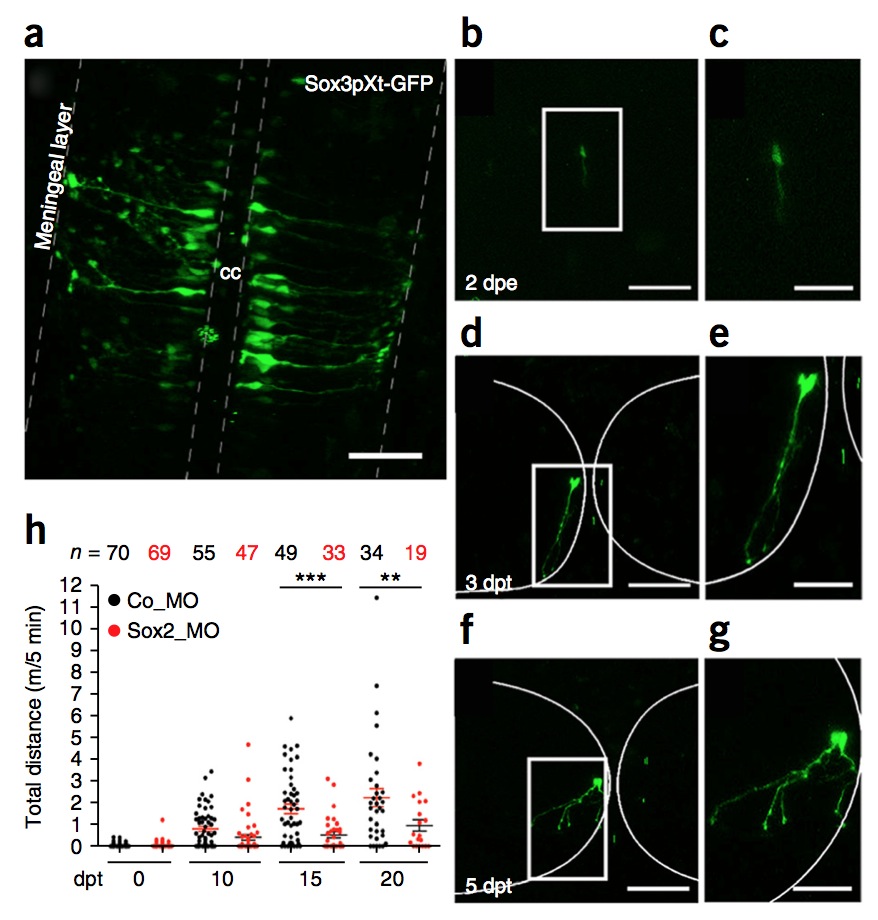
Figure 5 | Sox3pXt-GFP+ cells after spinal cord electroporation. (a) GFP expression 2 d after electroporation of Sox3pXt-GFP into the central canal (cc) of the larvae spinal cord. The morphology clearly shows that GFP+ cells have radial glia morphology. (b–g) Z stack of in vivo imaging of Sox3pXt-GFP+ electroporated cells (b) 2 dpe (days after electroporation) and (d,f) 3–5 dpt (days after transection), showing differentiation to neuronal morphology. White boxed areas in b,d,f are the magnified areas indicated in c,e,g and show the neural morphology of the electroporated cells. (h) Trajectory of swimming records of larvae electroporated with control morpholino (Co_MO; black dots) and Sox2 morpholino (Sox2_MO; red dots). Results for swimming records were analyzed by one-way ANOVA and Bonferroni multiple comparison test. Error bars indicate the s.e.m.(**P < 0.01; ***P < 0.001, indicates statistically significant). The decrease in Sox2_MO electroporated animals is explained because of animal death specific to the knockdown of Sox2. Scale bars, 50 μm (a,b,d,f); 100 μm (c,e,g). Spinal cord electroporation and swimming behavior test in larvae were performed in compliance with the institutional Bioethics and Biosafety committee at the Pontificia Universidad Catolica de Chile. b–h adapted with permission from ref. 10, Elsevier.
Adapted with permission from Macmillan Publishers Ltd: Edwards-Faret et al. (2017).Spinal cord regeneration in Xenopus laevis. Nature Protocols 12, 372–389 (2017) doi:10.1038/nprot.2016.177, copyright (2017).
Last Updated: 2017-01-24