Nutritional control of thyroid morphogenesis through gastrointestinal hormones
Maki Takagishi, Binta Maria Aleogho, Masako Okumura, Kaori Ushida, Yuichiro Yamada, Yusuke Seino, Sayoko Fujimura, Kaoru Nakashima, Asako Shindo
Curr Biol. 2022 Apr 11;32(7):1485-1496.e4. doi: 10.1016/j.cub.2022.01.075. Epub 2022 Feb 22.
Click here to view article at Current Biology.
Click here to view article at PubMed.
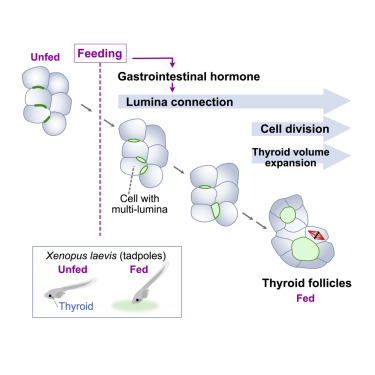
Highlights
• Thyroid morphogenesis requires external nutrients in Xenopus laevis
• Gastrointestinal hormones facilitate thyroid follicle enlargement
• Glucose-dependent insulinotropic polypeptide (GIP) induces thyroid follicle formation
• GIP receptor is required for normal thyroid morphogenesis in mice
Abstract
Developing animals absorb nutrients either through the placenta or from ingested food; however, the mechanisms by which embryos use external nutrients for individual organ morphogenesis remain to be elucidated. In this study, we assessed nutrient-dependent thyroid follicle morphogenesis in Xenopus laevis and investigated the role of secreted gastrointestinal (GI) hormones post-feeding. We found that feeding triggers thyroid follicle formation, and the thyroid cells showed transient inactivation of cell proliferation after feeding. In addition, the thyroid cells with multi-lumina were frequently observed in the fed tadpoles. The expression of the particular GI hormone incretin, glucose-dependent insulinotropic polypeptide (GIP), responded to feeding in the intestines of Xenopus tadpoles. Inhibition of dipeptidyl peptidase 4 (Dpp4), a degradative enzyme of incretin, increased the size of the thyroid follicles by facilitating follicular lumina connection, whereas inhibition of the sodium-glucose cotransporter (SGLT) reversed the effects of Dpp4 inhibition. Furthermore, injection of GIP peptide in unfed tadpoles initiated thyroid follicle formation-without requiring feeding-and injection of an incretin receptor antagonist suppressed follicle enlargement in the fed tadpoles. Lastly, GIP receptor knockout in neonatal mice showed smaller follicles in the thyroid, suggesting that the GI hormone-dependent thyroid morphogenesis is conserved in mammals. In conclusion, our study links external nutrients to thyroid morphogenesis and provides new insights into the function of GI hormone as a regulator of organ morphology in developing animals.
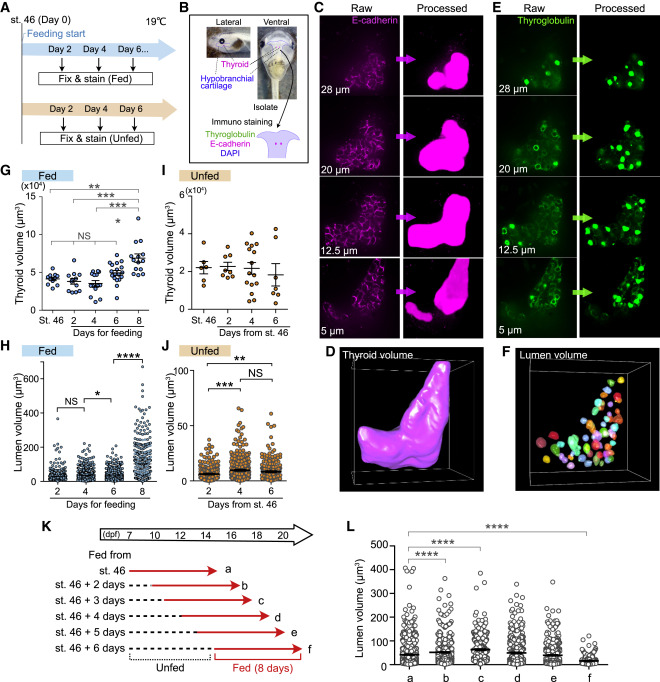
Figure 1. 3D image analyses reveal significance of external nutrients for thyroid morphogenesis
(A) Schematic illustration of the experimental schedule for feeding and immunostaining. For the fed group, the tadpoles were fed from st. 46 (day 0), and the thyroid tissues were isolated every 2 days (blue). The unfed tadpoles were incubated without feeding, and the thyroid tissues were isolated every 2 days after st. 46 (orange).
(B) The thyroid gland with hypobranchial cartilage was isolated and was immunostained with thyroglobulin and E-cadherin antibodies and 4′,6-diamidino-2-phenylindole (DAPI).
(C) Raw z stack images of E-cadherin staining and processed images using a deep learning model in Aivia software. The entire area of E-cadherin staining was filled to measure the thyroid volume.
(D) 3D images constructed with E-cadherin-stained processed images.
(E) Raw z stack images of thyroglobulin staining and processed images. The follicular luminal area was filled to measure the lumen volume.
(F) 3D images constructed with thyroglobulin-stained processed images.
(G) Measurement of thyroid volume of fed tadpoles (st. 46, 11 thyroids; day 2, 11 thyroids; day 4, 14 thyroids; day 6, 18 thyroids; day 8, 14 thyroids; ∗∗p = 0.0019, ∗∗∗p = 0.0002, ∗∗∗∗p < 0.0001, one-way ANOVA and Kruskal-Wallis test).
(H) Quantification of lumen volume of fed tadpoles (∗p = 0.018, ∗∗∗∗p < 0.0001, one-way ANOVA and Kruskal-Wallis test).
(I) Measurement of thyroid volume of unfed tadpoles (st. 46, 6 thyroids; day 2, 8 thyroids; day 4, 15 thyroids; day 6, 7 thyroids).
(J) Quantification of lumen volume (day 2, n = 235 lumina, mean = 6.214; day 4, n = 332 lumina, mean = 9.655; day 6, n = 246 lumina, mean = 8.31; ∗∗p = 0.0015, ∗∗∗p = 0.0001, one-way ANOVA and Kruskal-Wallis test).
(K) Scheme of the experimental schedule showing the days since the start of feeding. The lumen volumes of groups a–f (description given below) were measured. Red arrows indicate the feeding days.
(L) Quantification of lumen volume. a–f indicate the groups shown in (K) (a, n = 834 lumina, mean = 41.83; b, n = 600 lumina, mean = 51.79; c, n = 352 lumina, mean = 63.1; d, n = 418 lumina, mean = 49.3; e, n = 462 lumina, mean = 39.22, f, n = 323 lumina, mean = 16.15; ∗∗∗∗p < 0.0001, one-way ANOVA and Kruskal-Wallis test).
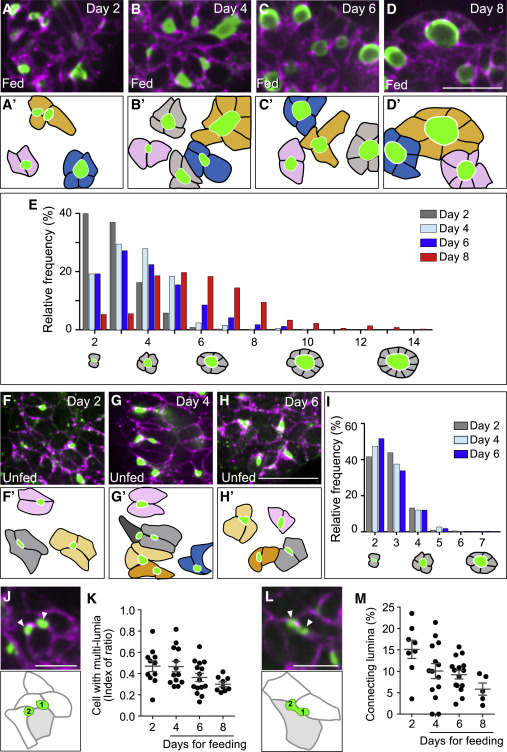
Figure 2. Food ingestion changes follicle topology
(A–D′) The immunostaining of fed tadpole thyroid tissues using thyroglobulin (green) and E-cadherin (magenta) antibodies. Day indicates the amount of feeding days, i.e., day 2 for 2 days (A and A′), day 4 (B and B′), day 6 (C and C′), and day 8 (D and D′). Outlines of single follicles were drawn in the lower panels (A′–D′); scale bar, 20 μm.
(E) Distribution of the cell numbers in single follicles on day 2 (gray), day 4 (light blue), day 6 (dark blue), and day 8 (red).
(F–H′) Immunostaining of thyroglobulin (green) and E-cadherin (magenta) antibodies in the thyroid tissues isolated from unfed Xenopus from 9 to 13 dpf. Outlines of each single follicle are shown in the lower panels (F′–H′). Scale bar, 20 μm.
(I) Cell numbers in individual follicles in unfed tadpoles (9 dpf, n = 219 follicles, mean = 2.749; 11 dpf, n = 405 follicles, mean = 2.709; 13 dpf, n = 275 follicles, mean = 2.665).
(J) Image of thyroglobulin (green) and E-cadherin (magenta) antibody staining of day 2 thyroid. Arrowheads indicate multi-lumina on a cell (filled with gray). Scale bar, 10 μm.
(K) Index of the number of cells with multi-lumina (J) (day 2, n = 11 thyroids, mean = 0.47; day 4, n = 13 thyroids, mean = 0.47; day 6, n = 15 thyroids, mean = 0.36; day 8, n = 9 thyroids, mean = 0.30).
(L) Image of thyroglobulin (green) and E-cadherin (magenta) antibody staining of day 2 thyroid. Arrowheads indicate connecting lumina on a cell (filled with gray). Scale bar, 10 μm.
(M) The ratio of the number of merging-like lumen (K) for the total number of lumina in a single thyroid (day 2, 8 thyroids, mean = 15.14; day 4, 14 thyroids, mean = 10.06; day 6, 15 thyroids, mean = 9.214; day 8, 5 thyroids, mean = 5.85).
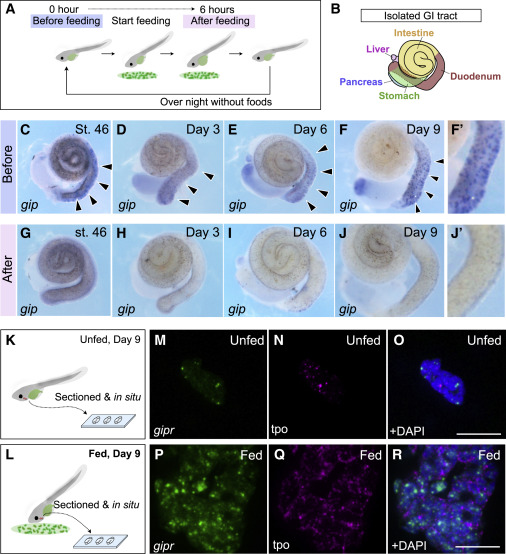
Figure 3. Glucose insulinotropic polypeptide (gip) expression responds to feeding in developing Xenopus, and its receptor is expressed in the thyroid
(A) Schematic illustration of collecting the GI tracts from the fed tadpoles. Tadpoles were fixed, and the GI tracts were isolated before and after feeding.
(B) GI tract of developing Xenopus tadpoles.
(C–J′) In situ hybridization of gip using Xenopus GI tubes. (C)–(F) are before feeding, whereas (G)–(J) are after feeding on stage (st.) 46, day 3, 6, and 9. (F′) and (J′) are magnified images of the duodenum from (F) and (J), respectively.
(K–L) Sample collection for RNAscope using sectioned thyroid tissues from the unfed (16 dpf) or fed tadpoles (16 dpf, 9 days of feeding from st. 46). Scale bar, 20 μm
(M–R) In situ hybridization using RNAscope to determine gipr expression in Xenopus thyroid tissues. (M)–(O) are the thyroids of unfed tadpoles, and (P)–(R) are the thyroids of fed tadpoles. tpo, thyroid peroxidase (thyroid marker). Scale bar, 20 μm.
Adapted with permission from Cell Press on behalf of Current Biology: Takagishi et al. (2022). Nutritional control of thyroid morphogenesis through gastrointestinal hormones. Curr Biol. 2022 Apr 11;32(7):1485-1496.e4. doi: 10.1016/j.cub.2022.01.075. Epub 2022 Feb 22.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2022-04-25