A regeneration-permissive environment requires myeloid cells following tail amputation
The myeloid lineage is required for the emergence of a regeneration-permissive environment following Xenopus tail amputation
Can Aztekin, Tom W. Hiscock, Richard Butler, Francisco De Jesús Andino, Jacques Robert, John B. Gurdon, Jerome Jullien
Development 2020 147: dev185496 doi: 10.1242/dev.185496 Published 5 February 2020
Click here to view article at Development.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
ABSTRACT
Regeneration-competent vertebrates are considered to suppress inflammation faster than non-regenerating ones. Hence, understanding the cellular mechanisms affected by immune cells and inflammation can help develop strategies to promote tissue repair and regeneration. Here, we took advantage of naturally occurring tail regeneration-competent and -incompetent developmental stages of Xenopus tadpoles. We first establish the essential role of the myeloid lineage for tail regeneration in the regeneration-competent tadpoles. We then reveal that upon tail amputation there is a myeloid lineage-dependent change in amputation-induced apoptosis levels, which in turn promotes tissue remodelling, and ultimately leads to the relocalization of the regeneration-organizing cells responsible for progenitor proliferation. These cellular mechanisms failed to be executed in regeneration-incompetent tadpoles. We demonstrate that regeneration incompetency is characterized by inflammatory myeloid cells whereas regeneration competency is associated with reparative myeloid cells. Moreover, treatment of regeneration-incompetent tadpoles with immune-suppressing drugs restores myeloid lineage-controlled cellular mechanisms. Collectively, our work reveals the effects of differential activation of the myeloid lineage on the creation of a regeneration-permissive environment and could be further exploited to devise strategies for regenerative medicine purposes.
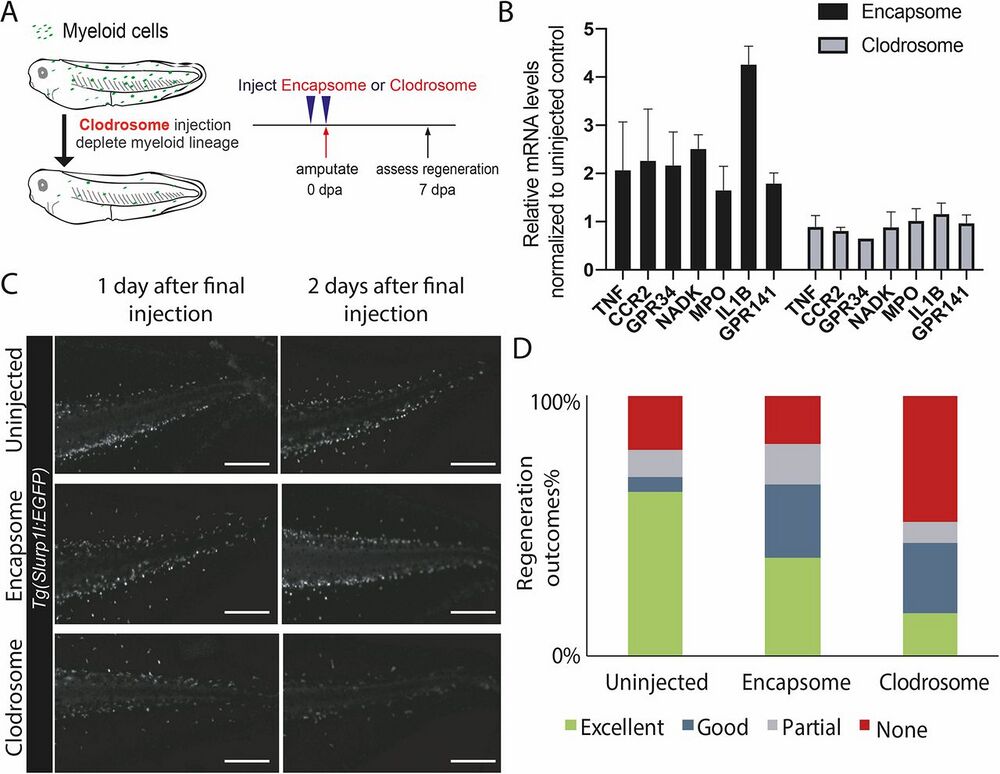
Fig. 1. Depleting the myeloid lineage impedes Xenopus tail regeneration. (A) Experimental design for assessing the effect of myeloid lineage depletion on regeneration. Regeneration-competent tadpoles were injected with either control (Encapsome), or clodronate-containing lipids (Clodrosome) before tail amputation. Regeneration efficiency was assessed at 7 days post-tail amputation (dpa). (B) Clodrosome injection decreases myeloid gene expression compared with Encapsome injection. Expression of myeloid lineage genes after Encapsome or Clodrosome injections was assessed by RT-qPCR analysis on amputated tails. All values were normalized to that of the uninjected controls. n≥3 biological replicates for each gene expression quantification, error bars represent s.e.m. (C) Representative images of Tg(slurp1l:EGFP) tadpoles post Encapsome or Clodrosome injection. A decrease in the slurp1l:EGFP myeloid lineage signal is particularly seen at dorsal and ventral vein regions. Scale bars: 250 μm. (D) Regeneration outcomes at 7 dpa in uninjected, Encapsome-injected or Clodrosome- injected regeneration-competent tadpoles. Samples were obtained from three biological replicates: uninjected n=30; Encapsome n=34; Clodrosome n=46.
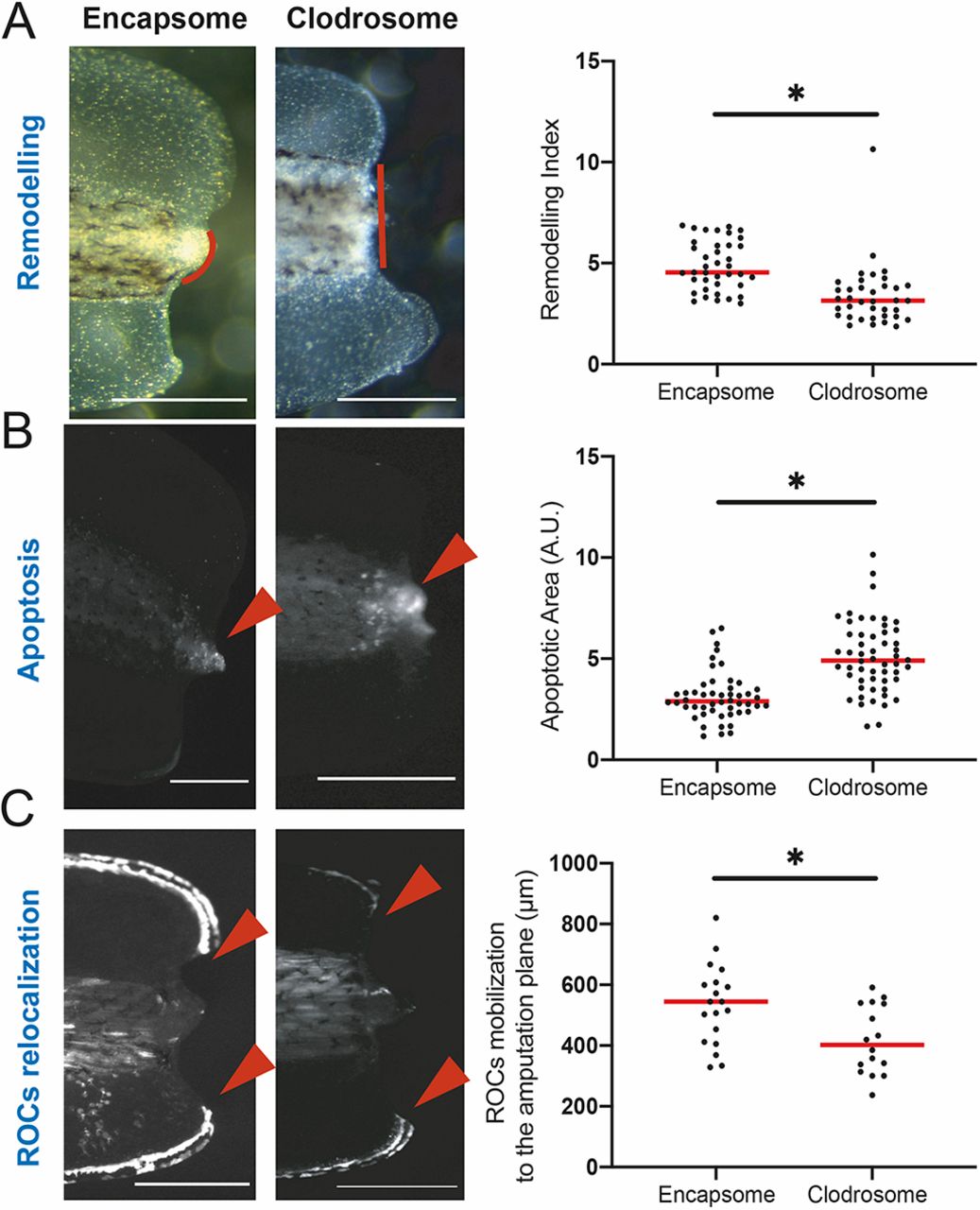
Fig. 2. The myeloid lineage is required for tissue remodelling, reduction of apoptosis levels, and relocalization of regeneration-organizing cells. (A) Left: Representative images of remodelling events occurring at 1 dpa in Encapsome- or Clodrosome-injected regeneration-competent tadpoles. Red lines indicate the posterior somitic region. Post-amputation histolysis and remodelling of tissue is impaired in Clodrosome-injected tadpoles. Scale bars: 250 μm. Right: Remodelling index quantification. All samples were obtained from at least three biological replicates: Encapsome n=39; Clodrosome n=37. Student’s t-test was used to assess statistical significance; *P<0.001. (B) Left: Representative images of apoptosis occurring at 1 dpa in Encapsome- or Clodrosome-injected regeneration-competent tadpoles. The red arrowheads indicate the location of the LysoSensor signal used to report apoptosis. Scale bars: 250 μm. Right: Apoptotic area quantification. All samples were obtained from at least three biological replicates: Encapsome n=49; Clodrosome n=53. Student’s t-test was used to assess statistical significance; *P<0.001. A.U., arbitrary unit. (C) Left: Representative images of ROC relocalization at 1 dpa for Encapsome- or Clodrosome-injected regeneration-competent tadpoles. The 7pbin:EGFP transgenic line was used to assess ROCs relocalization. Red arrowheads show the location of the leading ROC cells. Scale bars: 250 μm. Right: ROC relocalization quantification. All samples were obtained from at least three biological replicates: Encapsome n=19; Clodrosome n=16. Student’s t-test was used to assess statistical significance; *P<0.001. In graphs, each dot represents individual tadpoles; red line denotes median
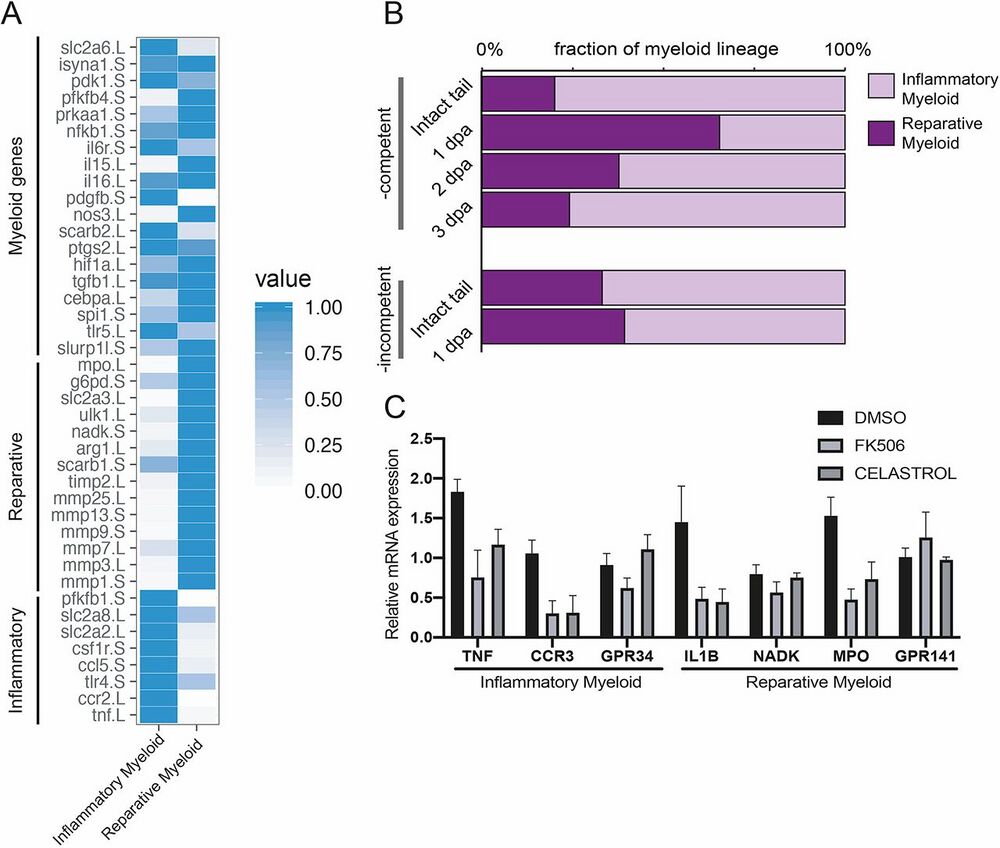
Fig. 4. A post-amputation shift from inflammatory to reparative myeloid activity characterizes regeneration- competent tadpoles. (A) Heat map showing known myeloid, reparative and inflammatory gene expression levels in the Xenopus tail cell atlas myeloid cell clusters. Values for each gene were normalized to that of the highest expressing cluster. Data
were re-analysed from Aztekin et al. (2019). (B) Relative abundance of inflammatory and reparative myeloid lineage cells post-amputation in regeneration-competent and -incompetent tadpoles. Data were re-analysed from Aztekin et al. (2019). (C) Myeloid cell cluster 1 and 2 gene expressions were tested 1 day after treatment of regeneration-incompetent tadpoles with the immune- suppressing drugs FK506 or Celastrol. n>3 biological replicates for each gene expression quantification; error bars represent s.e.m.
Adapted with permission from The Company of Biologists on behalf of Development: Aztekin et al. (2020). The myeloid lineage is required for the emergence of a regeneration-permissive environment following Xenopus tail amputation. Development 2020 147: dev185496 doi: 10.1242/dev.185496 Published 5 February 2020
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2020-09-10