Roles of NMDARs in Visual Circuit Development
Postsynaptic and Presynaptic NMDARs Have Distinct Roles in Visual Circuit Development.
Kesner P, Schohl A, Warren EC, Ma F, Ruthazer ES.
Cell Rep July 28, 2020; 32 (4): 107955.
Click here to view article at Cell Reports.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
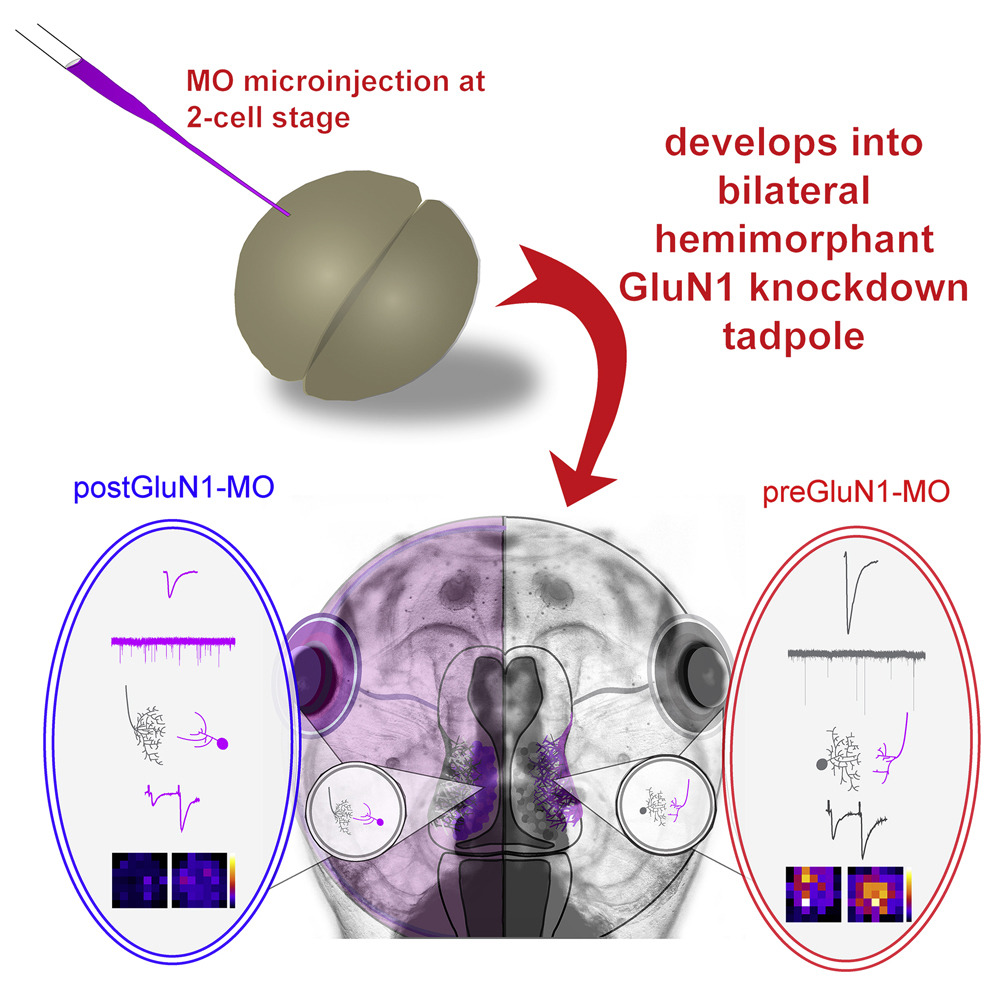
Highlights
• Presynaptic release properties are modulated by NMDARs on postsynaptic neurons
• Postsynaptic response amplitudes are influenced by NMDARs on presynaptic neurons
• NMDARs on post- and presynaptic neurons impact neuronal morphology in opposing ways
• NMDAR knockdown leads to abnormalities in visual input convergence
Abstract
To study contributions of N-methyl-D-aspartate receptors (NMDARs) in presynaptic and postsynaptic neurons of the developing visual system, we microinject antisense Morpholino oligonucleotide (MO) against GluN1 into one cell of two-cell-stage Xenopus laevis embryos. The resulting bilateral segregation of MO induces postsynaptic NMDAR (postNMDAR) knockdown in tectal neurons on one side and presynaptic NMDAR (preNMDAR) knockdown in ganglion cells projecting to the other side. PostNMDAR knockdown reduces evoked NMDAR- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated retinotectal currents. Although the frequency of spontaneous synaptic events is increased, the probability of evoked release is reduced. PreNMDAR knockdown results in larger evoked and unitary synaptic responses. Structurally, postNMDAR and preNMDAR knockdown produce complementary effects. Axonal arbor complexity is reduced by preNMDAR-MO and increased by postNMDAR-MO, whereas tectal dendritic arbors exhibit the inverse. The current study illustrates distinct roles for pre- and postNMDARs in circuit development and reveals extensive transsynaptic regulation of form and function.
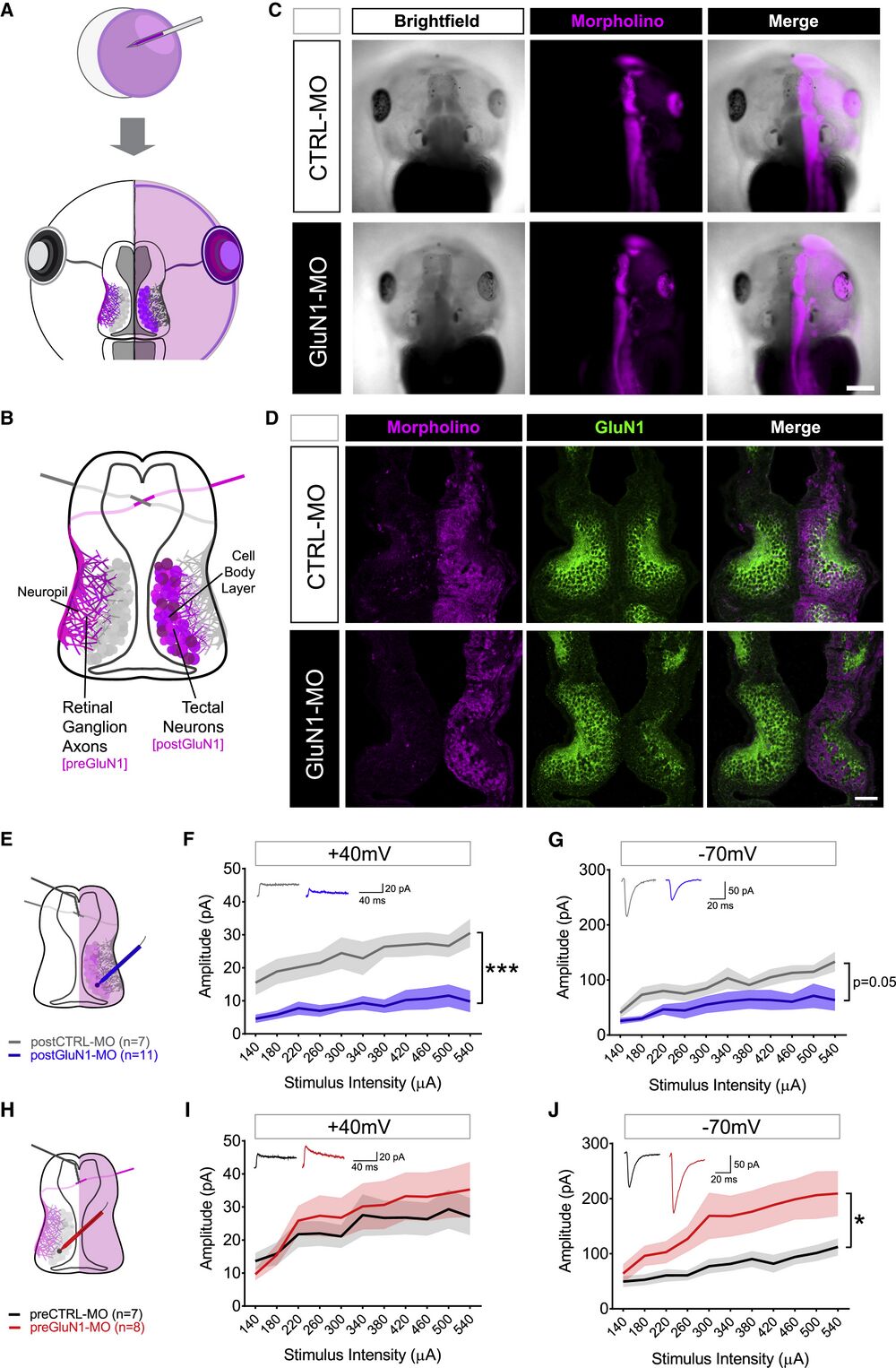
Figure 1
Hemimorphant Model to Study NMDARs on Post- and Presynaptic Neurons (A) Injection of the MO (magenta) into one cell at the two-cell stage of development produces tadpoles with the MO confined to one hemisphere. (B) Schematic of retinotectal system in a single hemimorphant tadpole with GluN1 KD in RGC axons in the left hemisphere (preGluN1-MO) and tectal neurons in the right hemisphere (postGluN1-MO). (C) Stage 45 tadpoles showing brightfield (left), MO-lissamine fluorescence (middle, magenta), and merged image (right). Scale bar, 500 μm. (D) Examples of confocal immunofluorescent sections of hemimorphant midbrains. First column: MO-lissamine fluorescence (magenta); second column: GluN1 immunofluorescence (green); third column: merged image. Scale bar, 100 μm. (E–G) Tectal neuron recordings in postGluN1-MO condition (E). Input-output curves of tectal neurons measured at a holding potential of +40 mV to ascertain the NMDAR-mediated current in postCTRL-MO (n = 7, gray) and postGluN1-MO (n = 11, blue) cells (F) and at −70 mV to ascertain the AMPAR-mediated current in postCTRL-MO (n = 7, gray) and postGluN1-MO (n = 11, blue) conditions (G). (H–J) Tectal neuron recordings in preGluN1-MO condition (H). Input-output curves of tectal neurons measured at a holding potential of +40 mV to ascertain the NMDAR-mediated current in preCTRL-MO (n = 7, black) and preGluN1-MO (n = 8, red) conditions (I) and at −70 mV to ascertain the AMPAR-mediated current in preCTRL-MO (n = 7, black) and preGluN1-MO (n = 8, red) conditions (J). n is number of cells. ∗p ≤ 0.05; ∗∗∗p ≤ 0.001, main effect by two-way repeated-measures ANOVA. Plots represent mean ± SEM.
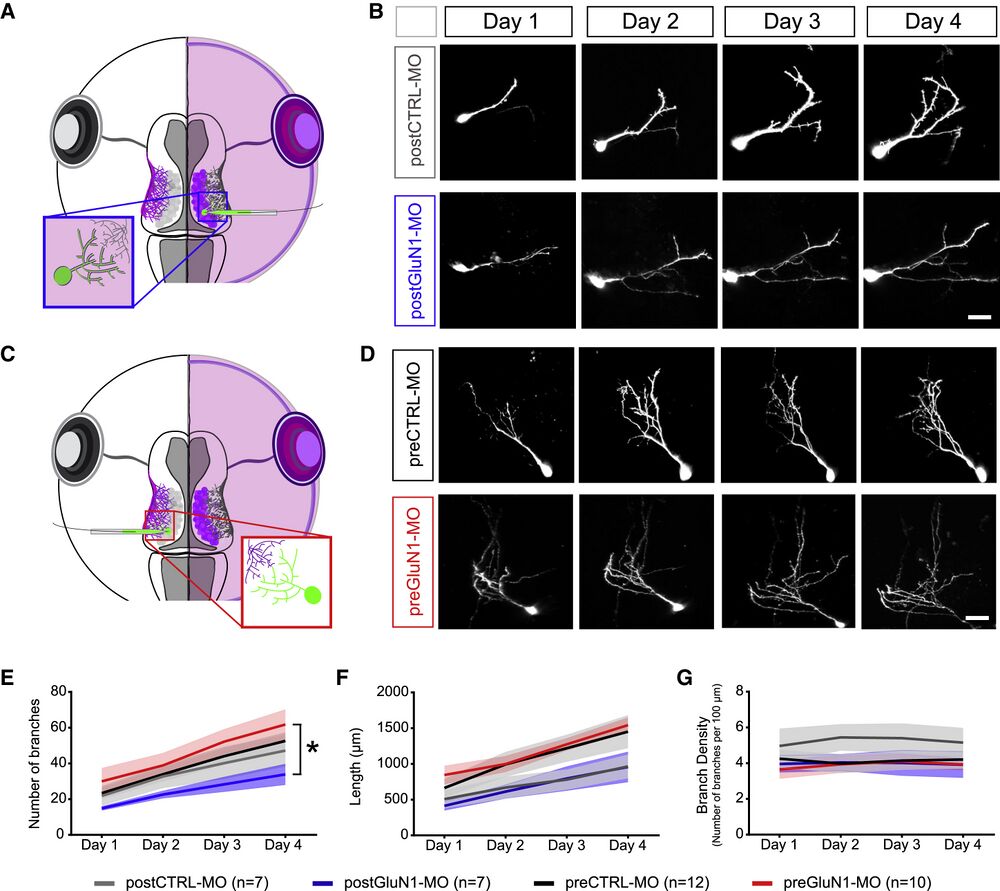
Figure 3
Knockdown of NMDARs in Post- and Presynaptic Neurons Differentially Affects Tectal Cell Dendritic Morphology (A) Single-cell electroporation of EGFP into the MO side of the tadpole labels tectal neurons in postCTRL-MO or postGluN1-MO conditions. (B) Example cells over 4 days of imaging for postCTRL-MO (top row) or postGluN1-MO conditions (bottom row). Scale bar, 20 μm. (C) Single-cell electroporation of EGFP into the non-MO side of the tadpole labels tectal neurons in preCTRL-MO or preGluN1-MO conditions. (D) Example cells over 4 days of imaging for preCTRL-MO (top row) or preGluN1-MO conditions (bottom row). Scale bar, 20 μm. (E–G) Morphometric analysis of dendritic branch number (E), total dendritic arbor length (F), and branch density (number of branches per 100 μm) (G) over 4 days (postCTRL-MO [n = 7, gray], postGluN1-MO [n = 7, blue], preCTRL-MO [n = 12, black], and preGluN1-MO [n = 10, red]). Because pre- and postCTRL-MO groups were not significantly different from each other, they were grouped to allow a comparison of postGluN1-MO and preGluN1-MO conditions. n is number of cells. ∗p ≤ 0.05, main effect by two-way repeated-measures ANOVA. Plots represent mean ± SEM.
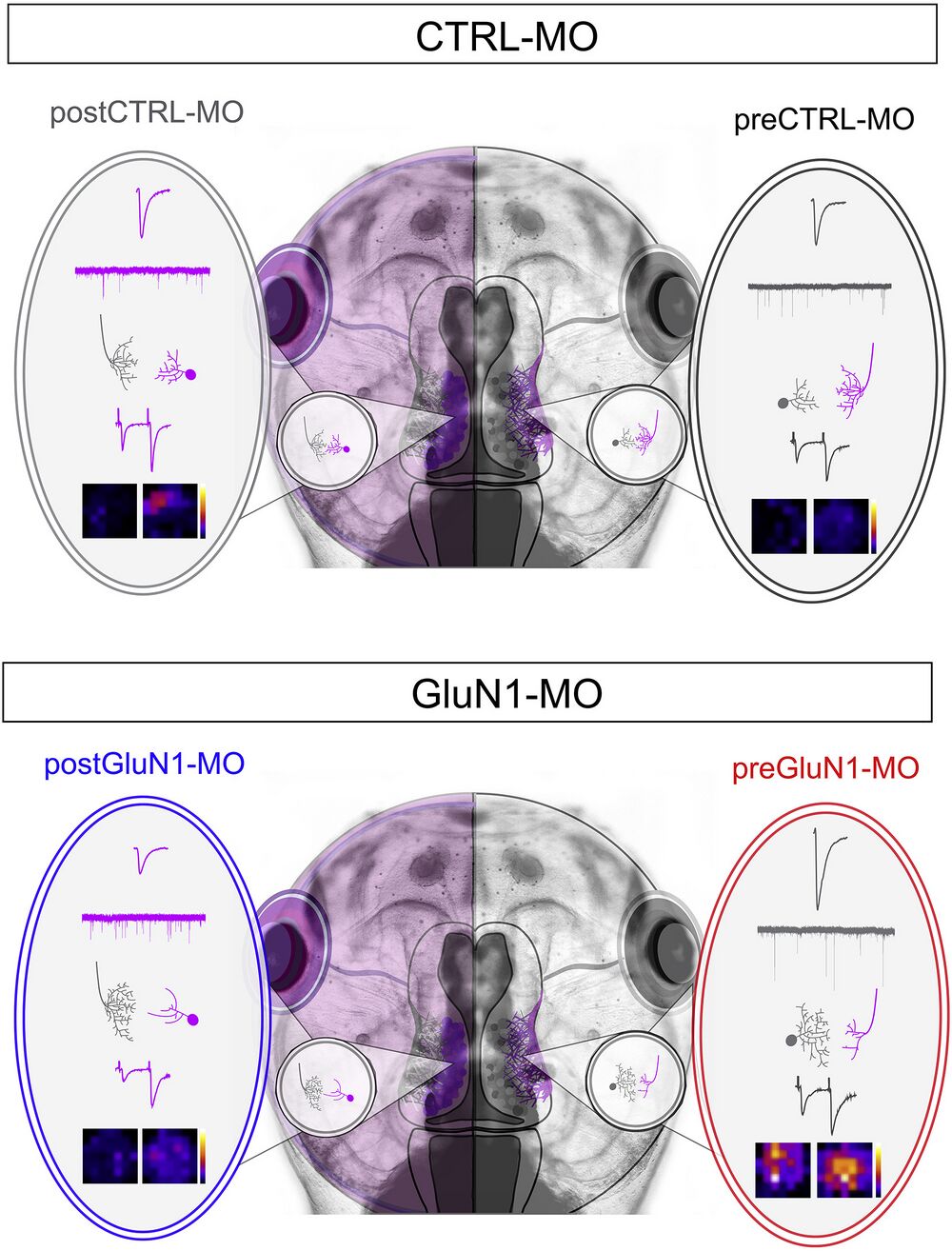
Figure 7
Overall Summary of the Findings PostGluN1-MO leads to smaller AMPAR-mediated responses, higher mEPSC frequency, and simplified tectal dendritic arbors with more complex RGC arbors, as well as a larger PPR. PreGluN1-MO leads to larger AMPAR-mediated responses, higher amplitude of mEPSCs, and simplified RGC arbors with more complex tectal dendritic arbors, as well as an overall more responsive receptive field.
Adapted with permission from Elsevier on behalf of Cell Reports: Kesner et al. (2020). Postsynaptic and Presynaptic NMDARs Have Distinct Roles in Visual Circuit Development. Cell Rep July 28, 2020; 32 (4): 107955. DOI:https://doi.org/10.1016/j.celrep.2020.107955
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2020-08-18