ATAC-seq in Xenopus
Chromatin Accessibility and Histone Acetylation in the Regulation of Competence in Early Development
Melody Esmaeili, Shelby A Blythe, John W Tobias, Kai Zhang, Jing Yang, Peter S Klein
Dev Biol. Feb 28, 2020 [Online ahead of print]. In press.
https://doi.org/10.1016/j.ydbio.2020.02.013
Click here to view article at Developmental Biology.
Click here to view article at Pubmed.
Click here to view article on Xenbase.
Highlights
• ATAC-Seq in Xenopus laevis identifies open chromatin domains at the gastrula stage.
• Putative cis-regulatory sites in gastrulae are enriched for pluripotency factor binding motifs.
• Dorsal Wnt target gene promoters are not accessible at the early gastrula stage.
• Histone deacetylases mediate loss of competence at dorsal Wnt target genes.
• Mechanisms for loss of competence are context-dependent.
Description
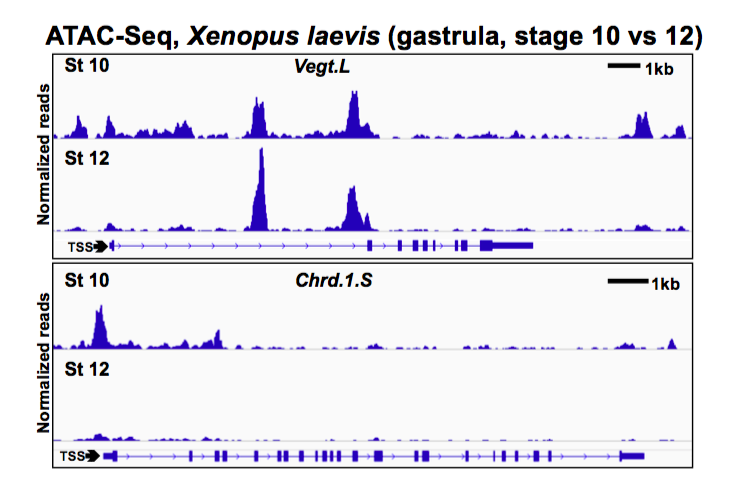
Assay for Transposase-Accessible Chromatin followed by sequencing (ATAC-seq) in Xenopus laevis ectodermal explants identified ~70,000 accessible regions at early (stage 10) and late (stage 12) gastrula stage. Overlap with p300 ChIP-seq data (from Session et al) identified >22,000 putative cis-regulatory modules (pCRMs). Accessibility was reduced at 279 annotated promoters and > 1800 promoter-distal pCRMs by the end the gastrula stage; these pCRMs were frequently near promoters for transcription factors that regulate early development and motif analysis of pCRMs showed marked enrichment of pluripotency factor binding sites (for Sox, Oct, and KLF factors). Promoters for dorsal genes (sia1, sia2, and nodal3.1) that have lost competence to respond to Wnt signaling are not accessible at the early gastrula stage but competence can be maintained by inhibiting HDACs and increasing histone acetylation at these promoters, supporting the hypothesis that competence to respond to dorsal Wnt signaling is regulated at the level of chromatin structure.
Abstract
As development proceeds, inductive cues are interpreted by competent tissues in a spatially and temporally restricted manner. While key inductive signaling pathways within competent cells are well-described at a molecular level, the mechanisms by which tissues lose responsiveness to inductive signals are not well understood. Localized activation of Wnt signaling before zygotic gene activation in Xenopus laevis leads to dorsal development, but competence to induce dorsal genes in response to Wnts is lost by the late blastula stage. We hypothesize that loss of competence is mediated by changes in histone modifications leading to a loss of chromatin accessibility at the promoters of Wnt target genes. We use ATAC-seq to evaluate genome-wide changes in chromatin accessibility across several developmental stages. Based on overlap with p300 binding, we identify thousands of putative cis-regulatory elements at the gastrula stage, including sites that lose accessibility by the end of gastrulation and are enriched for pluripotency factor binding motifs. Dorsal Wnt target gene promoters are not accessible after the loss of competence in the early gastrula while genes involved in mesoderm and neural crest development maintain accessibility at their promoters. Inhibition of histone deacetylases increases acetylation at the promoters of dorsal Wnt target genes and extends competence for dorsal gene induction by Wnt signaling. Histone deacetylase inhibition, however, is not sufficient to extend competence for mesoderm or neural crest induction. These data suggest that chromatin state regulates the loss of competence to inductive signals in a context-dependent manner.
Processed ATAC-seq data viewable on Xenbase JBrowse coming soon.
Last Updated: 2020-04-14