Evolutionarily conserved Tbx5-Wnt2/2b pathway orchestrates cardiopulmonary development
Proc Natl Acad Sci USA November 6, 2018; 115 (45): E10615-E10624.
Steimle JD, Rankin SA, Slagle CE, Bekeny J, Rydeen AB, Chan SS, Kweon J, Yang XH, Ikegami K, Nadadur RD, Rowton M, Hoffmann AD, Lazarevic S, Thomas W, Boyle Anderson EAT, Horb ME, Luna-Zurita L, Ho RK, Kyba M, Jensen B, Zorn AM, Conlon FL, Moskowitz IP .
Click here to view article at PNAS.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Abstract
Codevelopment of the lungs and heart underlies key evolutionary innovations in the transition to terrestrial life. Cardiac specializations that support pulmonary circulation, including the atrial septum, are generated by second heart field (SHF) cardiopulmonary progenitors (CPPs). It has been presumed that transcription factors required in the SHF for cardiac septation, e.g., Tbx5, directly drive a cardiac morphogenesis gene-regulatory network. Here, we report instead that TBX5 directly drives Wnt ligands to initiate a bidirectional signaling loop between cardiopulmonary mesoderm and the foregut endoderm for endodermal pulmonary specification and, subsequently, atrial septation. We show that Tbx5 is required for pulmonary specification in mice and amphibians but not for swim bladder development in zebrafish. TBX5 is non-cell-autonomously required for pulmonary endoderm specification by directly driving Wnt2 and Wnt2b expression in cardiopulmonary mesoderm. TBX5 ChIP-sequencing identified cis-regulatory elements at Wnt2 sufficient for endogenous Wnt2 expression domains in vivo and required for Wnt2 expression in precardiac mesoderm in vitro. Tbx5 cooperated with Shh signaling to drive Wnt2b expression for lung morphogenesis. Tbx5 haploinsufficiency in mice, a model of Holt-Oram syndrome, caused a quantitative decrement of mesodermal-to-endodermal Wnt signaling and subsequent endodermal-to-mesodermal Shh signaling required for cardiac morphogenesis. Thus, Tbx5 initiates a mesoderm-endoderm-mesoderm signaling loop in lunged vertebrates that provides a molecular basis for the coevolution of pulmonary and cardiac structures required for terrestrial life.
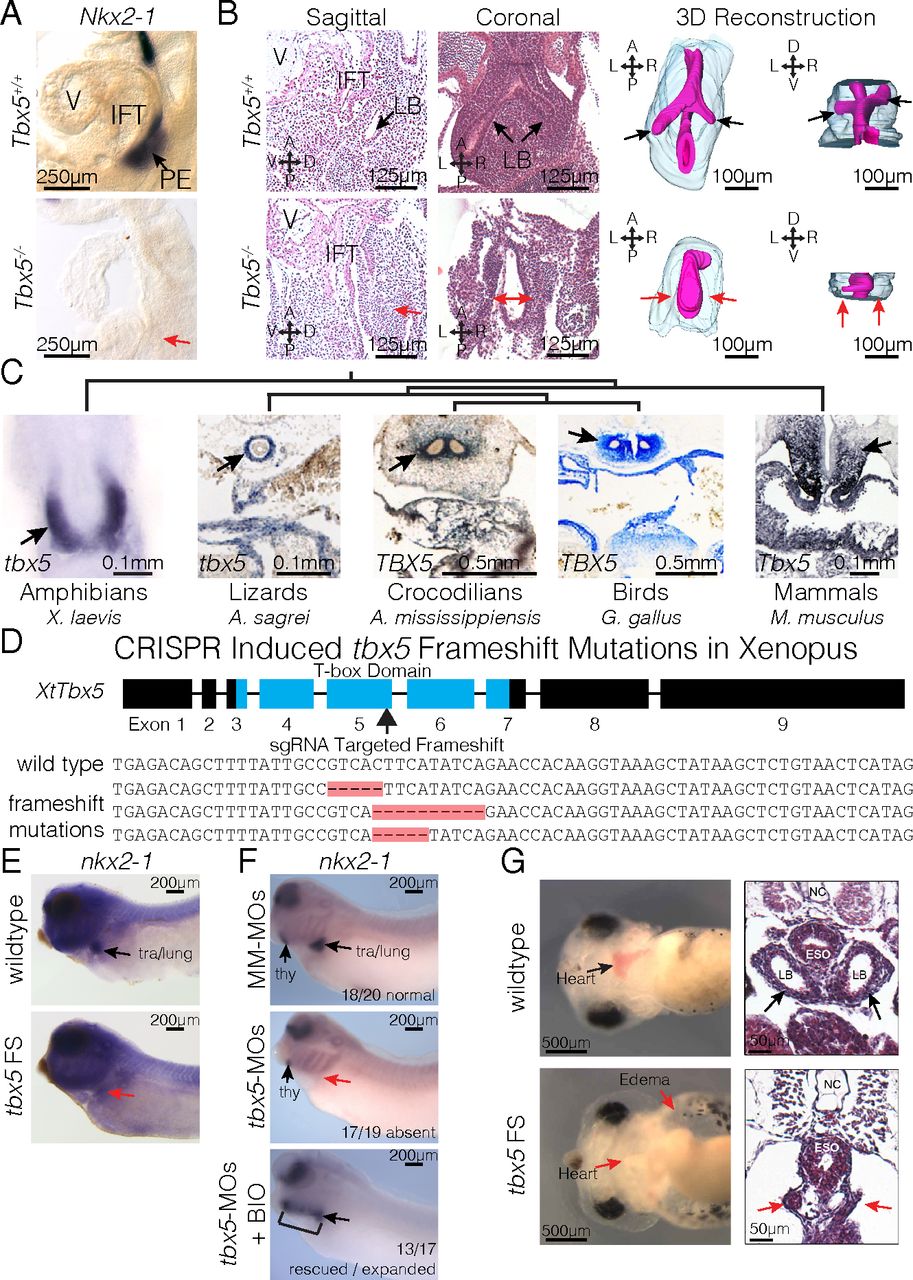
Fig. 2. Tbx5 is required for lung development in mice and frogs. (A) RNA ISH for Nkx2-1 in E9.5 Tbx5+/+ and Tbx5−/− embryos. The PE, inflow tract (IFT), and cardiac ventricle (V) are labeled. (B) Histology from both sagittal and coronal perspectives (Left) and 3D reconstruction (Right) of E10.5 lungs from Tbx5+/+ and Tbx5−/− embryos. Black and red arrows point to the lung bud (LB) branches or lack of branches off the foregut. (C) ISH stains of Tbx5 across vertebrate species. Arrows indicate expression in the mesodermal derivatives surrounding the PE. (D, Upper) Strategy for generating biallelic frameshift mutations using sgRNA targeted to the fifth exon of X. tropicalis to disrupt the T-box domain. (Lower) Examples of sequences recovered from tbx5 FS mutants. (E and F) RNA ISH of NF35 tadpoles for nkx2-1 in wild type and tbx5 FS mutants (E) and tadpoles injected with mismatched morpholinos (MM-MOs), tbx5-MOs, or tbx5-MOs cotreated with BIO (F). (G) Live images (Left) and H&E-stained transverse sections (Right) of NF42 tadpoles, depicting anatomical defects induced by CRISPR-mediated mutation of tbx5.
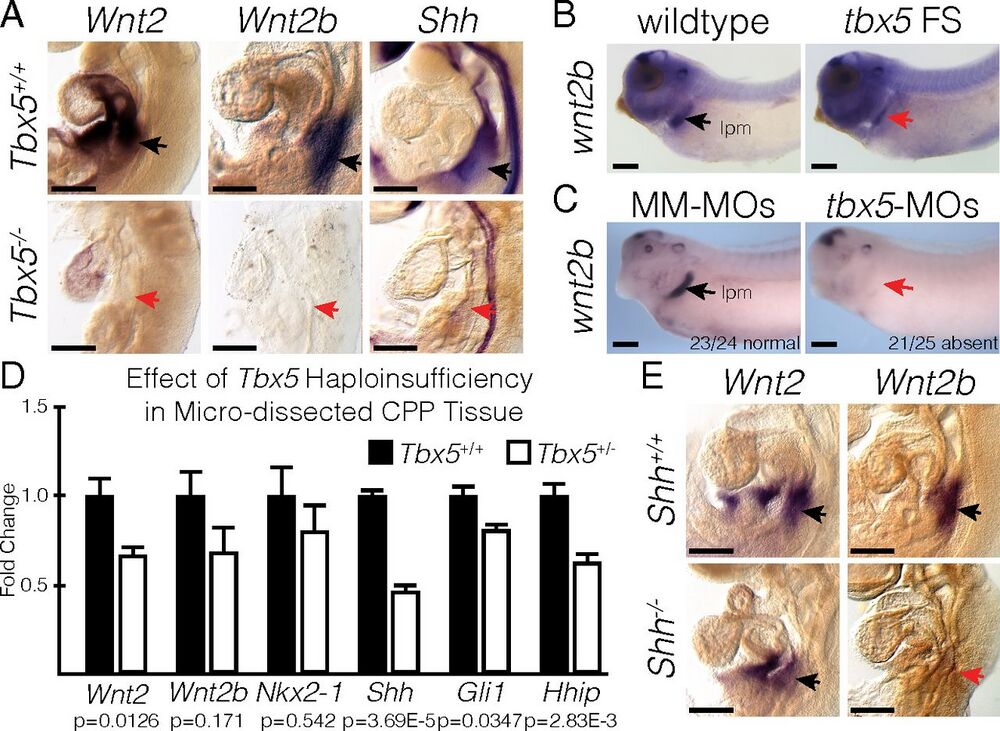
Fig. 3. Tbx5 is required for Wnt2/2b and Shh expression. (A) RNA ISH for Wnt2, Wnt2b, and Shh in E9.5 Tbx5+/+ and Tbx5−/− mouse embryos. Black and red arrows point to positive and negative staining, respectively, in the lung-forming region. (B and C) RNA ISH for wnt2b performed in wild-type or tbx5 FS NF35 tadpoles (B) and in NF35 tadpoles injected with mismatched morpholinos (MM-MOs) or tbx5-MOs (C). The stained region corresponds with the lateral plate mesoderm (lpm). (D) qRT-PCR of microdissected CPP tissue from E9.5 Tbx5+/+ or Tbx5+/− mouse embryos. (E) RNA ISH for Wnt2 and Wnt2b in E9.5 Shh+/+ and Shh−/− mouse embryos. (Scale bars: 250 µm.)
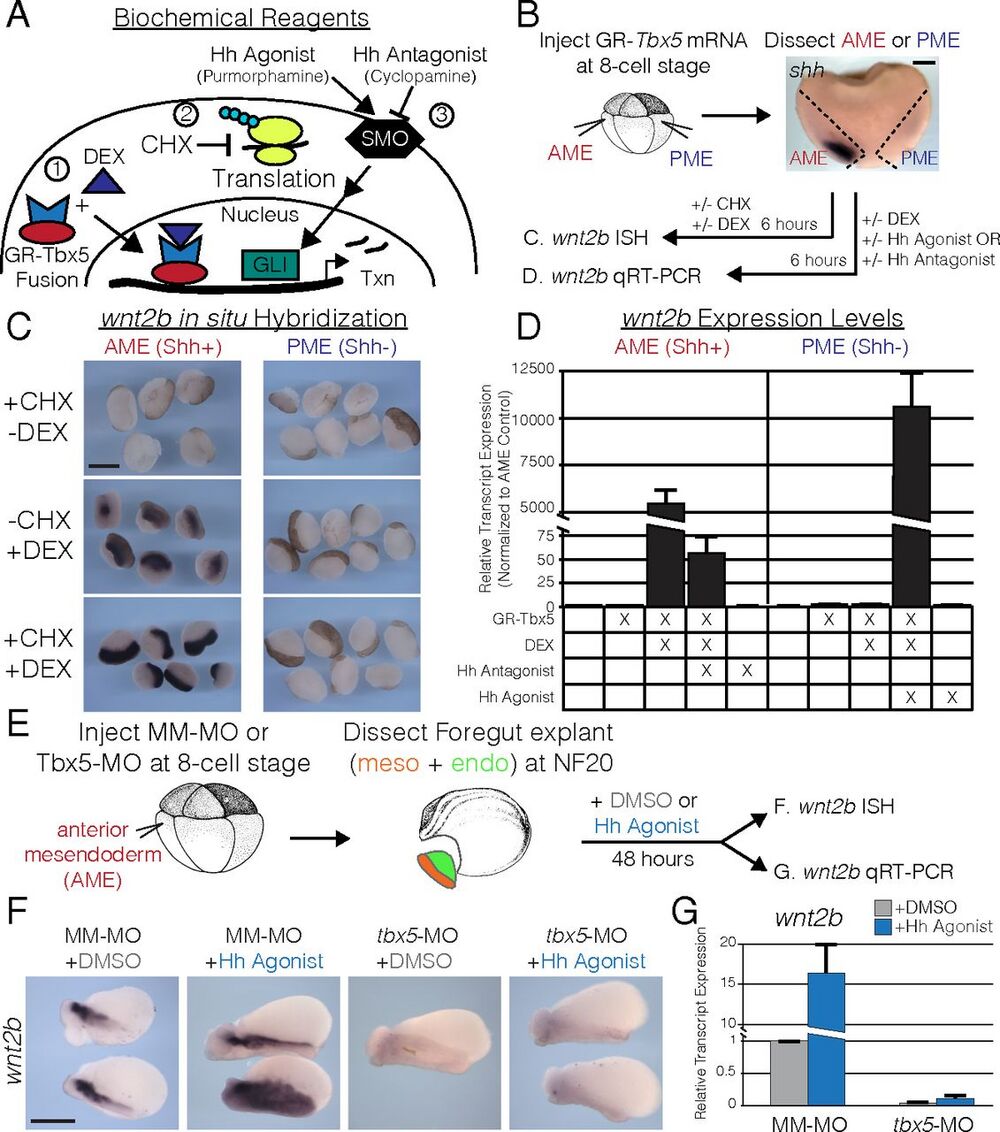
Fig. 4. tbx5 directly regulates wnt2b expression for lung development in the presence of Hh signaling. (A) To study the interaction of Tbx5 and Hh signaling, we utilized (1) a DEX-inducible GR-Tbx5 fusion protein; (2) CHX to inhibit protein synthesis; and (3) pharmacological agonists (purmorphamine) and antagonists (cyclopamine) of Smoothened (SMO) to activate or repress Hh signaling. (B) Strategy used in C and D for examining the regulation of wnt2b by Tbx5 in the presence or absence of Shh signaling in Xenopus. The AME (red) corresponds to shh-expressing tissue, and the PME (blue) corresponds to shh-negative tissue. (C) RNA ISH for wnt2b in AME or PME explants treated with CHX, DEX, or both. Note that the ISH signal is black; the brown color is pigment. (D) qRT-PCR of wnt2b in AME or PME explants, with or without GR-Tbx5, and treated with combinations of DEX, Hh agonist, and Hh antagonist. (E) Strategy used in F and G to examine the regulation of wnt2b by Hh signaling in the presence or absence of tbx5. AME explants were treated with DMSO (gray) or the Hh agonist purmorphamine (blue) for 48 h. (F) RNA ISH for wnt2b on DMSO- or Hh agonist-treated explants from embryos injected with mismatched morpholinos (MM-MO) or tbx5-MO. (G) qRT-PCR of wnt2b normalized to DMSO-treated MM-MO–injected explants. (Scale bars: B, 200 µm; C and E, 400 µm.)
Adapted with permission from the National Academy of Sciences on behalf of PNAS: Steimle et al. (2018). Evolutionarily conserved Tbx5-Wnt2/2b pathway orchestrates cardiopulmonary development. Proc Natl Acad Sci USA November 6, 2018; 115 (45): E10615-E10624. Copyright (2018).
Last Updated: 2018-11-15