Proteomics of fertilization in the Xenopus egg
Proteomics of phosphorylation and protein dynamics during fertilization and meiotic exit in the Xenopus egg.
Marc Stuart Presler, Elizabeth Van Itallie, Allon M. Klein, Ryan Kunz, Peg Coughlin, Leonid Peshkin, Steven Gygi, Martin Wühr, Marc Kirschner
Click here to view the PREPRINT of this article.
Click here to view the PNAS Early Edition of this article.
Published online before print November 28, 2017, doi: 10.1073/pnas.1709207114
PNAS November 28, 2017
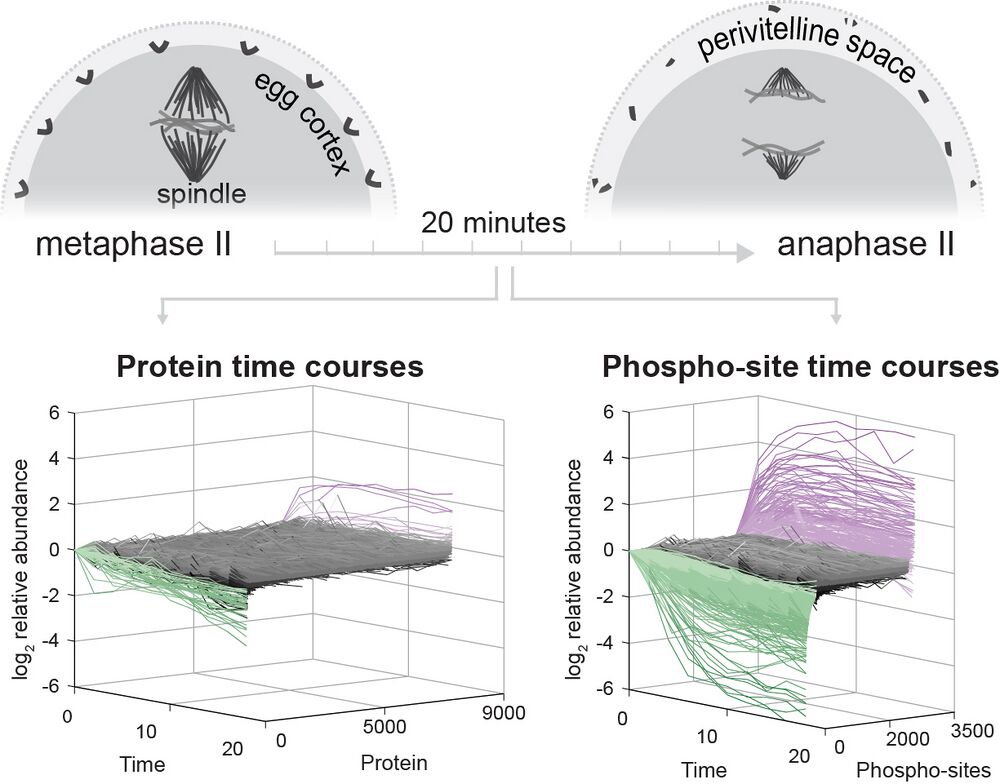
The fertilization response in Xenopus laevis eggs has served an important role in the study of the cell cycle and other early events like the blocks to polyspermy. These rapid and essential processes are driven by the regulation of existing proteins mainly through degradation (e.g., Cyclin B) and phosphorylation. The regulation of protein function depends on the quantitative features of these posttranslational modifications, such as absolute rates and stoichiometry of modifications. This study presents a quantitative view of the posttranslational economy of the events following fertilization using new methods for systematically determining phosphosite occupancy. This allows for absolute quantification and improved interpretability of post-translational modification analysis. The study presents principles of protein regulation in meiotic exit, the slow block to polyspermy, nuclear pore complex reassembly, calcium signaling, and more. The new approaches are applicable broadly to study of protein biochemistry in the developing embryo.
Abstract
Fertilization releases the meiotic arrest and initiates the events that prepare the egg for the ensuing developmental program. Protein degradation and phosphorylation are known to regulate protein activity during this process. However, the full extent of protein loss and phospho-regulation is still unknown. We examined absolute protein and phospho-site dynamics of the fertilization response by mass spectrometry-based proteomics in electro-activated eggs. To do this, we developed a new approach for calculating the stoichiometry of phospho-sites from multiplexed proteomics that is compatible with dynamic, stable and multi-site phosphorylation. Overall, the data suggest that degradation is limited to a few low abundance proteins. However, this degradation promotes extensive dephosphorylation that occurs over a wide range of abundances during meiotic exit. We also show that eggs release a large amount of protein into the medium just after fertilization, most likely related to the blocks to polyspermy. Concomitantly, there is a substantial increase in phosphorylation likely tied to calcium activated kinases. We identify putative degradation targets and components of the slow block to polyspermy. The analytical approaches demonstrated here are broadly applicable to studies of dynamic biological systems.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
